with hints and answers to problems
First Semester
Units 2-22
![]()
with hints and answers to problems
First Semester
Units 2-22
![]()
These are the problems for your assignments. Please do them for each Unit assignment.
Important Charts and Tables
Quick Unit Finder
Do not click here until the page is completely loaded!!!
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
*** Here Beginneth The Unit Assignments: ***
Assignments must be done in INK!!
Questions must be answered in COMPLETE SENTENCES, and the METHOD OF SOLUTION must be shown for the Calculations (the Hup, Two, Three, Four)!
Assignments must be headed properly!
Assignments will not be graded if the above is not done.
It is best to do these assignments ON-LINE, because they have hot links to charts, tables, problems, and hints.
For research, go to The On-Line Reference Text or use your Text Book.
Note: You need not print the Assignment sheet, just answer the questions in complete sentences and solve the problems with the Hup, Two, Three, Four.
NO problems for Unit 1, just read Chapter 1, Introduction to Chem, in the on-line Text Book.
Unit 2, Measuring & Calculating
ALL WORK MUST BE DONE IN INK!!!
For research, go to The On-Line Reference Text Unit 2. Measuring & Calculating
1. How many significant digits are there in each of the following?
a. 903.2, b. 90.3, c. 900.04, d. 0.0090, e. 0.0900, f. 99, g. 0.0088, h. 0.049, i. 0.02, j. 70.
Ans: a =4, b =3, c =5, d =2, e =3, f =2, g =2, h =2, i =1, j= 2.
2.
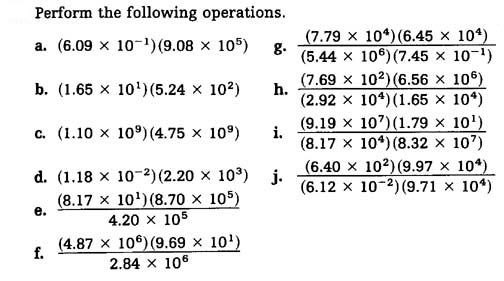
Ans: a = 5.53 X105, b = 8.65 X 103, c = 5.23 X 1018, d = 2.60 X 101, g = 1.24 X 103, h = 1.05 X 101
METHOD MUST BE SHOWN FOR ALL PROBLEMS!-
3. What is the density of a piece of cement which has a mass of 8.76 g and a volume of 3.07 cm3? Ans: 2.85 g/cm3
4. What is the density of a piece of cork which has a mass of 0.650 g and a volume of 2.71 cm3? Ans: 0.240 g/cm3.
5. Limestone has a density of 2.72 g/cm3. What is the mass of 981 cm3 of limestone? Ans: 2670 g.
Solve the following problems for mass:
6. Ammonium magnesium chromate has a density of 1.84 g/cm3. What is the mass of 6.96 cm3 of this substance? Ans: 12.8 g.
7. Barium perchlorate has a density of 2.74 g/cm3. What is the mass of 610 cm3 of this substance? Ans: 1670 g.
Solve the following problems for volume:
8. Cerium sulfate has a density of 3.17 g/cm3. What is the volume of 706 g of this substance? Ans: 223 cm3.
ALL EXPLANATIONS MUST BE IN COMPLETE SENTENCES!!
9. Why is mass used instead of weight for scientific work?
10. Why is it important to maintain the correct number of significant digits in calculations?
11. List the number of significant digits for each of the following:
a. 1.x108, b. 6.8 x 108, c. 4.930 00 x 109, d. 8.420 000 0 x 108
Ans: a =1, b =2, c =d, d =8
12. Express in scientific notation:
a. 36.8, b. 0.0387, c 0.000 216 5, d. 516 830 000 000
Ans: a = 3.6 X 101, b = 3.87 X 10-2, c = 2.165 X 10 -4, d = 5.16 X 1011
For research, go to The On-Line Reference Text Unit 3. Matter
EXPLAIN YOUR ANSWERS!
1. Classify the following materials as heterogeneous mixtures, solutions, compounds, or elements.
a. air, b. trail mix, c. paper, d. table salt, e. alcohol, f. apple, g. milk, h. plutonium, i. water.
2. Indicate how you would demonstrate that each of the following is a heterogeneous mixture or a homogeneous mixture:
a. a piece of lumber, b. a glass of soda pop, c. a piece of cloth advertised as 50% wool and 50% synthetic.
3. Classify the following properties as chemical or physical.
a. color, b. reactivity, c. flammability, d. odor, e. porosity, f. stability, g. ductility, h. solubility, i. expansion, j. melting point, k. rusting, l. reacts with air
4. Classify the following changes as chemical or physical:
a. digestion of food, b. fading of dye in cloth, c. growth of a plant, d. melting of ice.
5. Classify the following changes as chemical or physical:
a. explosion of gasoline in an automobile engine, b. formation of clouds in the air.
6. Make a list of five solutions commonly found in the home.
7. Classify the following materials as heterogeneous mixture, solution, compound, or element.
a. paint, b. copper, c. granite, d. leather, e. corn syrup, f. gold.
8. Classify the following properties as chemical or physical:
a. density, b. melting point, c. length.
For research, go to The On-Line Reference Text Unit 4. Chemical Formulas
Click here to print the Chemical Formulas Assignment and Practice Sheet.pdf.
... These are Chemical Formulas to be done and turned in. Keep the handout for future practice.
Unit 5 will be done after Unit 6.
For research, go to The On-Line Reference Text Unit 6. Chemical Equations.
Print the Equations & Answers.pdf Assignment and Practice Sheet.
... These are Chemical Equations to be done and turned in. Keep the handout for future practice.
For research, go to The On-Line Reference Text Unit 5. The Mole
1. Calculate the molecular or formula masses of the following compounds, all in amu (g/mol):
a. C2H6, b. SiCl4, c. MgCO3, d. Ca3(P04)2, e. K2S, f. CH2CHCH2OH, g. Pb3(As04)2, h. C12H22011. Ans: a=30, b=170, c=84, d=310, e=110, f=58, g=899, h=342.
Make the following conversions SHOWING YOUR METHOD, the Hup, Two, Three, Four!
2. 1.00 x 1026 molecules of SnCl2 to moles. Ans: 1.66 X 102 mol.
3. 0.400 moles of H2O to molecules. Ans: 2.41 X 1023 molecules.
4. 76.0 grams CaBr2 to moles. Ans: 0.380 mol. Or 3.80 X 10-1 mol.
5. 18.0 grams HBr to moles. Ans: 0.222 mol. Or 2.22 X 10-1 mol.
6. 9.30 moles SiH4 to molecules. Ans: 5.60 X !024 molecules.
7. Find the mass of one atom of Na. Ans. 3.82 X 10-23g/atom
8. Find the mass of one molecule of H2SO4. Ans. 1.63 X 10-22 g/molecule
Compute the molarity of the following solutions:
9. 145 g NH4Cl in 500 ml of solution. Ans: 5.4 M
10. 41.3 g Fe(N03)2 in 100 ml of solution. Ans: 2.3 M
11. 35.0 g MnSiF6 in 50.0 ml of solution. Ans: 3.56 M
SHOW YOUR METHOD, the Hup, Two, Three, Four!
Describe the preparation of the following solution:
12. 500 ml of 1.50 M AgF. Ans: Dissolve 95.3 g of AgF in enough water to make 500ml of solution.
Find the percentage composition of the following:
13. CsF. Ans: 87.5%; 12.5%.
14. Bi203. Ans: 89.7%, 10.3%.
15. BaH2. Ans: 98.6%, 1.44%.
Find the empirical formulas of the following compounds:
16. l.67 g Ce, 4.54 g I. Ans: CeI3
17. 6.27 g Ca, 1.46 g N. Ans: Ca3N2
18. The molecular mass of benzene is 78 and its empirical formula is CH. What is the molecular formula for benzene? Ans: C6H6
19. What is the molecular formula of dichloroacetic acid, if the empirical formula is CHOCl and the molecular mass is 129g/mol? Ans: C2H2O2Cl2
Find the formulas for the following hydrates:
20. 95.3 g LiNO3, 74.7 g H2O. Ans: LiNO3•3H20
21. 89.2% BaBr2, 44.6% H2O (Note: %-ages may be replaced with grams because they are in the same ratio). Ans: BaBr2•8H20
22. Explain the difference between the terms mole and molarity.
23. Explain the difference between an empirical formula and a molecular formula.
Unit 7, Stoichiometry (math of Big Chem)
For research, go to The On-Line Reference Text Unit 7. Quantitative Relationships
Note: You must have a balanced equation before solving the problems!
1. How many grams of H2 can be produced from the reaction of 11.5 grams of sodium with an excess of water? Hint: 2Na + 2H2O ---> 2NaOH + H2. Ans: 0.505g .
2. Nitrogen reacts with 2.OO grams of hydrogen. How many grams of ammonia are produced? Hint: Ammonia is NH3. Nitrogen is diatomic. Ans: 11.2g.
3. How many grams of oxygen are required to burn 85.6 grams of carbon? Hint: Oxygen is diatomic, C + O2 ---> CO2. Ans: 228g.
4. The action of carbon monoxide on iron(III) oxide (ferric oxide) can be represented by the equation, Fe2O3 + 3CO ----> 2Fe + 3CO2. What would be the amount of carbon monoxide used if 18.7 grams of iron were produced? Ans: 14.1g.
5. How many grams of hydrochloric acid are required to react with 75.1 grams of calcium hydroxide? Remember the rules for parentheses for calcium hydroxide. Ans: 74.6g.
6. How many grams of hydrogen gas are produced when 5.62 grams of aluminum react with hydrochloric acid? Hint: hydrochloric acid is hydrogen chloride, hydrogen gas is diatomic. Ans: 0.631g.
Table A-5 will be used for Problems 7, 8, 9.
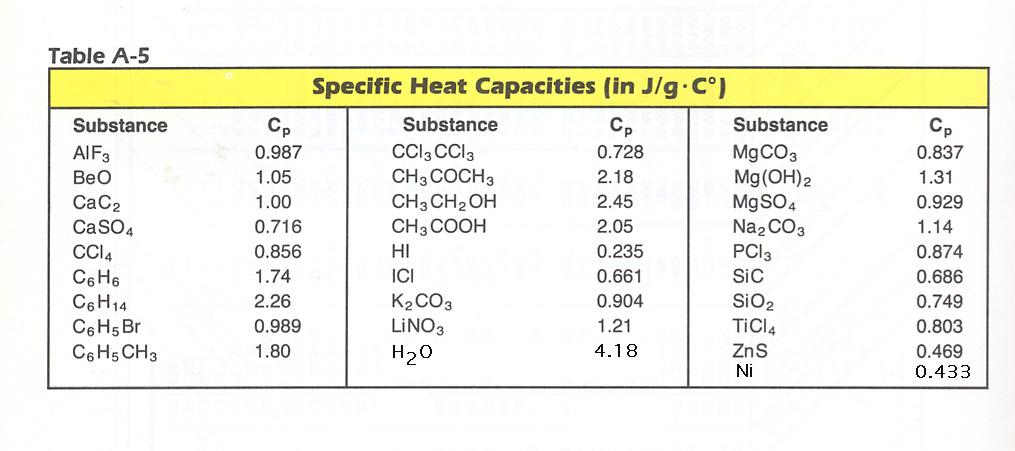
7. How much heat is required to raise the temperature of 91.4 g of PCl3 from 25.OoC to 76.1oC? Ans. = 4080 J.
8. How much heat is required to raise the temperature of 4.66 g CCl4 from 20.9oC to 76.8oC? Ans. = 223 J
9. How much heat is required to raise the temperature of 787 g of H2O from 18.OoC to 100.OoC? Ans. = 270,OOO J.
10. Compute the change in enthalpy for the formation of 193 grams of ammonium bromide from ammonia and hydrogen bromide. Table A-6 . Hint: Remember the difference between ammonia and ammonium (nevah forget!), and find ΔH then multiply by the moles of ammonium bromide. Ans: ΔH = -188 kj/mol, -370 kJ.
11. Compute the change in enthalpy for the displacement of O.O663 grams of bromine from sodium bromide by chlorine. Table A-6 . Note: Sodium chloride is also formed. Hint: Bromine is diatomic. Ans: ΔH = -102 kj/mol, -0.0423 kJ.
12. What is the difference between endothermic reactions and exothermic reactions?
13. Give an example of the use of activation energy in everyday life.
For research, go to The On-Line Reference Text Unit 8. Atomic Structure
1. State the five steps of Dalton's Atomic Theory.
2. What did each of the following contribute in forming the atomic theory?
a. Dalton, b. Thomson, c. Rutherford, d. Chadwick, e, Avogadro, f. Franklin, g. Faraday, h. Crookes, i. Perrin, j. Millikan, k. Roentgen, l. Becquerel, m. Curie.
3. State the Law of Conservation of Mass and give an example.
Hint: A = the mass number = protons + neutrons. Z = the atomic number = the number of protons. Z also = the number of electrons in a neutral atom. The number of neutrons = A - Z.
4. A particular atom of argon contains 18 protons, 18 electrons, and 22 neutrons. What is the atomic number of this atom? What is its mass? Ans: 18, 40g/mol.
5. What are the basic differences among protons, neutrons, and electrons?
6. How many electrons, neutrons, and protons are in the isotope of nitrogen with mass number 14? Ans: 7 electrons, 7 protons, 7 neutrons.
7. State the Law of Definite Proportions and give an example.
8. State the Law of Gay Lussac and give an example (balance equation).
9. Why is the Induction Coil (Sparky) important in Chemistry.
10. List five properties of the Cathode Rays in the Crookes Tubes.
11. Describe an experiment to show that electrons have mass.
12. What is discovered in the Tube of Sir JJ Thompson?
13. What three things were measured in the Millikan Oil Drop Experiment?
14. Define Ion and give two examples.
15. Define Isotope and give an example.
16. What are two chemistry things we learn from X-Rays?
17. How was Radioactivity discovered?
18. What are the three particles of radioactivity?
19. List five properties of radioactivity.
20. How was Fluorescence discovered?
For research, go to The On-Line Reference Text Unit 9. Electron Clouds/Probability
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
Electron Orbitals Probability Diagrams
1. What is the wavelength of an electron of mass 9.11 x 10-28 kg traveling at a velocity of 2.00 x 108 m/s? (Planck's constant = 6.63 x 10-34 J/Hz. Hint: Substitute in this formula:
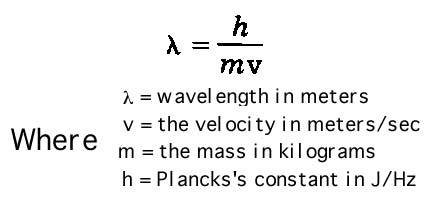
Ans: 3.64 x 10-15m.
For electron configurations problems, use the Electronic Energy Levels (Goose Chart).
2. Calculate the maximum number of electrons that can occupy the levels when n = 2, 3, 5, and 7.
3. How many orbitals are in a(n) a. d sublevel, b. f sublevel ?
4. Write the electron configurations of the elements with Z = 1 through Z = 20. (Z = atomic number = the number of electrons to configure) . Hint: 1s2 2s2 2p6 3s2 3p6 4s2 ... etc.
5. Write electron configurations and draw the dot diagrams for the following elements: Remember that dot structures use the valence (outermost) electrons and s and p orbitals only.
a. Z = 28, b. Z = 18, c. Z = 16, d. Z = 47, e. Z = 19, f. Z = 32.
6. What is the relationship between momentum and wavelength?
7. How many electrons can exist in the fifth energy level? See Electronic Energy Levels.
8. What elements are composed of atoms having the following configurations:
Hint: the total number of electrons, Z, is the atomic number. Use The Periodic Table to determine the element.
a. 1s2 2s2 2p6 3s2 3p6 4s2 3d5
b. 1S2 2S2 2p6 3S2 3p6 4S2 3d10 4p6 5S2 4d4
9. Write the electron configurations for niobium and zinc.
10. How many pairs of electrons are there in an atom of boron? an atom of sulfur? an atom of fluorine? Hint: A filled orbital represents a pair of electrons on the Electronic Energy Levels.
Unit 10, Periodic Table. Unit 11 is included with Unit 10.
For research, go to The On-Line Reference Text Unit 10. Periodic Table and Unit 11. Periodic Properties.
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
Use The Periodic Table of the Elements for the following problems:
1. Classify the following elements as metals, metalloids, or nonmetals:
a. cadmium, b. calcium, c. californium, d. carbon, e. dysprosium, f. praseodymium, g. thallium.
2. Are there more metals or nonmetals in the periodic table?
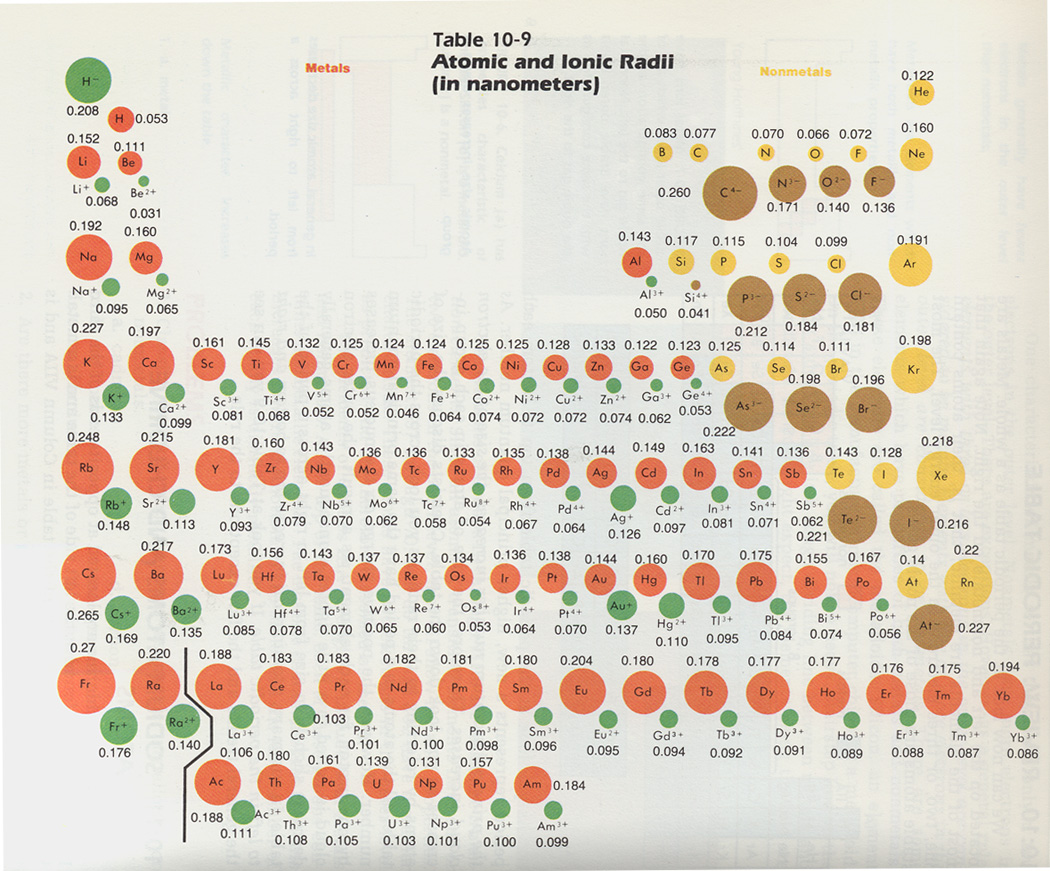
3. From each of the following pairs of particles, select the particle which is larger in radius: Use Table 10-9 below.
a. He, Ne. b. Be, B. c. N, N3-, d. Al, Al3+. e. P, As. f. S, Br. g. K, Ca. h. Se, Se2+. i. Sc, Sc3+.
b. State the reasons for your answers.
4. Predict the oxidation numbers for gadolinium, radium, and niobium. Hint: Remember that columns are families having the same oxidation number (valence), and the grand tendency of the elements.
5. Classify the following elements as metals or nonmetals:
a. chromium, b. fluorine, c. gold, d. helium, e. iron, f. neodymium, g. nitrogen, h. tantalum, i. hydrogen, j. lithium, k. phosphorus, l. silicon.
Use Table 10-9 above for the following:
6. What happens to the size of the atoms as you move from left to right across a horizontal row of the periodic table?
7. What happens to the size of the atoms as you move from the top to the bottom of a column of the periodic table?
8. How does the size of a positive ion compare to the size of the atom from which it was formed?
9. How does the size of a negative ion compare to the size of the atom from which it was formed?
10. Element X has the following electron configuration ls2 2s2 2p6 3s2 3p6 4s2 3104p4
a. Locate its position on the periodic table. Hint: count up the electrons for the Atomic Number.
b. To what group and to what period does this element belong?
c. Classify the element as a metal, nonmetal, or metalloid.
d. List the properties associated with the classification you chose.
e. Predict the oxidation number of the element (its valence).
f. Draw the electron dot diagram for an atom of Element X. Draw the electron dot diagram for an ion of Element X. Hint: a positive ion has lost electrons, a negative ion has gained electrons. And dot structures use outermost electrons s and p orbitals only. Ah Yaz!
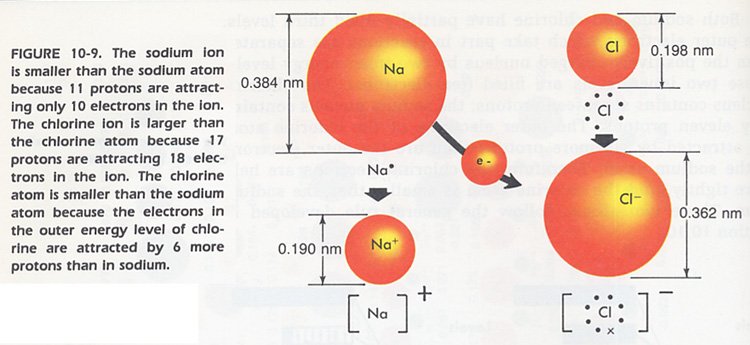
g. How does the size of an atom of Element X compare to that of an ion of Element X? Hint: adding electrons increase the size of an ion, removing electrons decreases the size of an ion. See Figure 10-9 below.
11. The positions of some elements are highlighted on the periodic table, Figure l0-12 below. For each element highlighted answer the following:
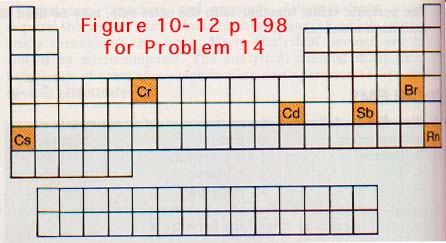
a. To what group and what period does this element belong?
b. Classify the element as a metal, nonmetal, or metalloid.
c. List the properties this element should exhibit based on the classification you chose in b.
d. Write the electron configuration of the element.
e. Predict the oxidation number of the element.
f. Draw the electron dot diagram for both an atom and an ion of this element.
Units 12 & 13 Chemical Bondage and Molecular Structure.
For research, go to The On-Line Reference Text Unit 12. Chemical Bonding and 13. Molecular Structure
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
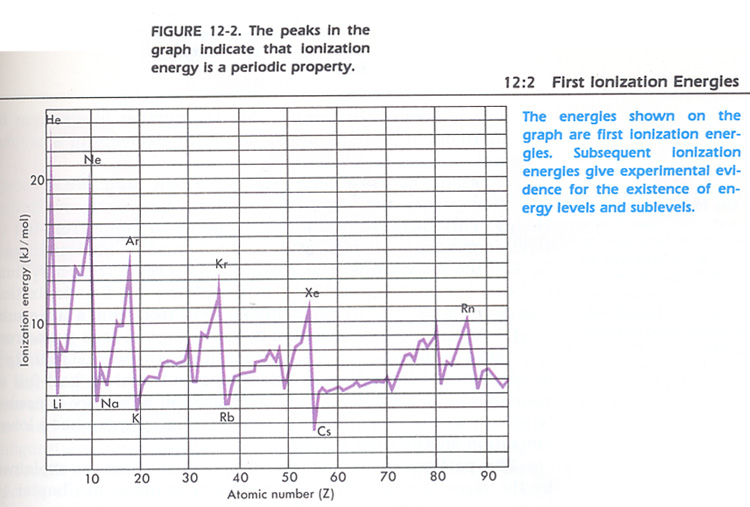
1. Explain each of the peaks in the graph in Figure 12-2. Hint: see which group of elements are at each peak.
2. Classify the bonds between the following pairs of atoms as principally ionic or covalent: Hint: remember how these bonds are formed.
a. boron and carbon, b. cesium and fluorine, c. fluorine and silicon, d. hydrogen and chlorine, e. magnesium and nitrogen, f. beryllium and fluorine, g. bromine and strontium, h. chlorine and lithium, i. chlorine and sodium, j. hydrogen and iodine.
3. For each atom pair listed below, decide whether an ionic or a covalent bond would form between the elements: Hint: see where they are located on the The Periodic Table of the Elements.
a. fluorine - astatine, b. boron - thorium, c. gadolinium - astatine, d. lanthanum- selenium, e. strontium - chlorine, f. iodine- sodium.
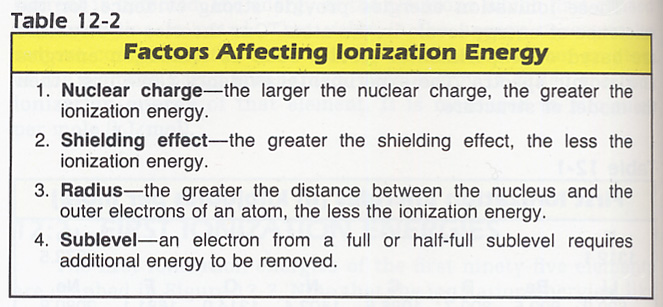
4. Explain the differences in the six ionization energies of carbon shown in Table 12-3 below. Hint: Factors Determining Ionization Energy are in Table 12-2 below. And consider the Ratio of remaining electrons to the nuclear charge (prisoners/guard ratio).
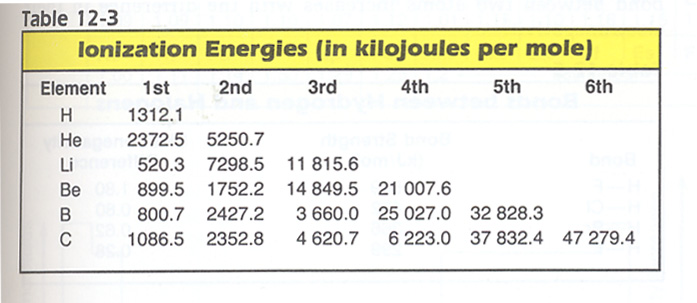
5. Using Tables 12-8 & 12-9, predict the bond lengths indicated for the substances: Hint: take the average.
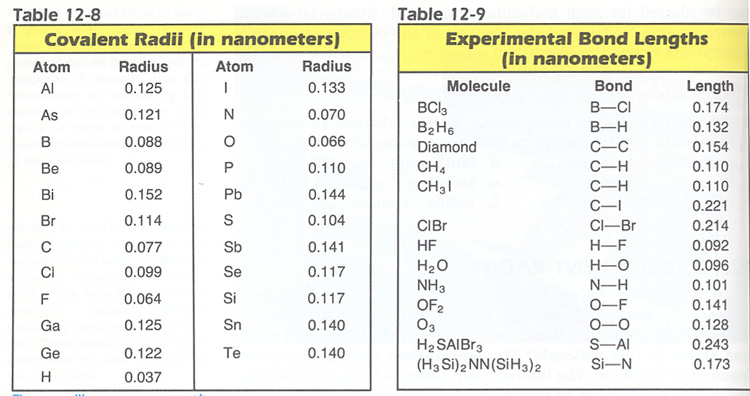
a. Cl--CI in Cl2, b. N--H in NH3, c. H--Br in HBr, d. F--O in OF2.
6. What four factors affect the values obtained for ionization energies of an element. See Table 12-2 above.
7. Predict the bond angles indicated in the following compounds: Hint: Use Fig. 13-1 and Tables 13-1,2 and The Periodic Table of the Elements. If you know the bond angles for one element, they will be the same for other elements in the same column.
Figure 13-1
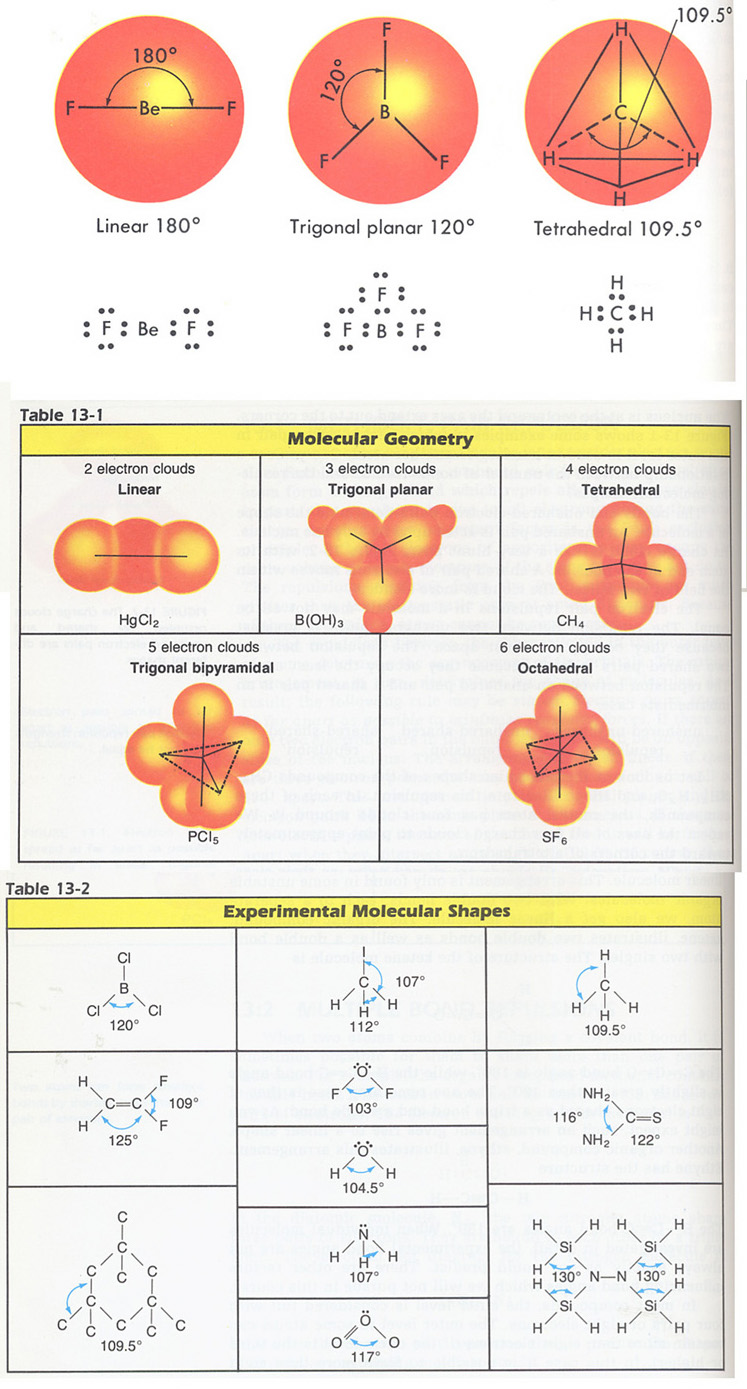
a. H--Te--H in H2Te, b. H--P--H in PH3, c. Cl--As--Cl in AsCl3.
8. What is the major difference between sigma, s , and pi, p , bonds? See Figures 13-6,7.
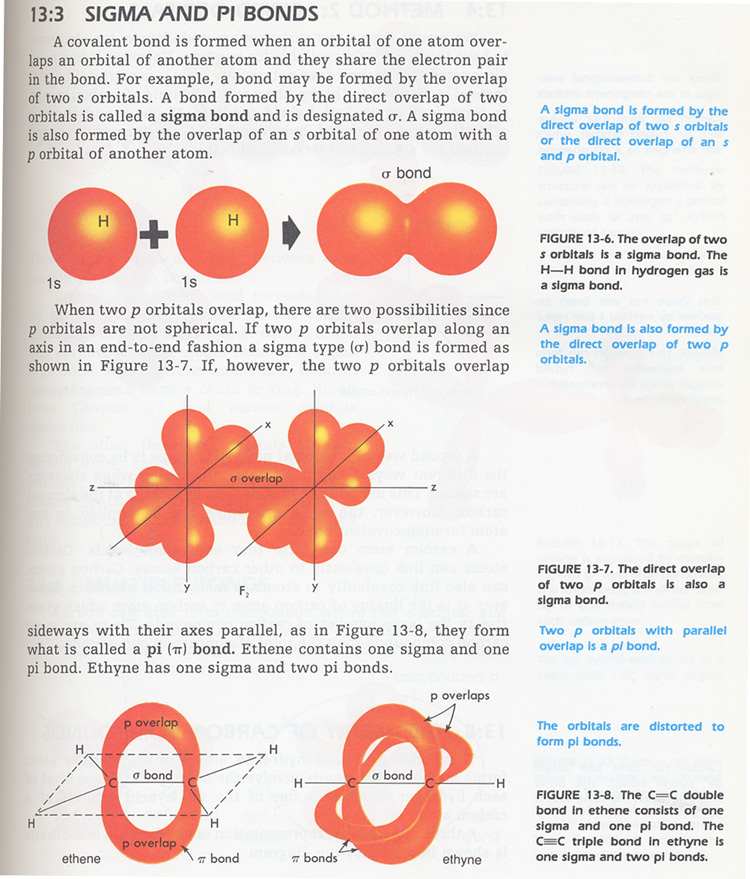
9. What is meant by the term hybridization? Hint: certain electrons are promoted from s to p orbitals.
10. Predict the shapes of the following molecules: Hint: Use Fig 13-1, Tables 13-1,2 above and The Periodic Table of the Elements. If you know the bond angles for one element, they will be the same for other elements in the same column.
a. SO2, b. BF3, c. SF6, d. SF4.
11. One might expect the bond angle for each C--H bond in methane (CH4) to be 90o. Why is this prediction incorrect? See Figure 13-1 above. Hint: Think in three dimensions (x, y, z axes).
12. Draw Boxes and Dot structures for a. NF3, b. H2S, c. N2.
13. Explain the Intermolecular Force (van der Waals) and give an example.
14. Explain the Hydrogen Bond and give an example.
15. Explain the Metallic Bond and give an example.
16. Explain Infra-red Spectroscopy and what we learn from it.
17. Name the Four Modes of Molecular Vibrations. Hint: Modes of Molecular Vibrations.
For research, go to The On-Line Reference Text Unit 14. Polar Molecules
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
1. Define Polar Molecule and give an example.
2. Define the Hydrogen Bond and give an example.
3. What are Intermolecular Forces (van der Waals) and how do they compare with covalent bonds in strength?
4. What is the difference between an Intermolecular Forces and a Covalent Bond?
5. What is a Hydrogen Bond and how does it compare in strength with a Covalent Bond?
6. Define Symmetric & Asymmetric Molecules. Why are they important?
7. Define Allotrope, name two allotropes of oxygen and write their formulas.
8. What is the difference between the molecular structure of liquid water and solid water?
9. Why does ice float in water?
10. Why is water polar?
11. How does microwave cooking work?
12. What is chromotography and how is it used?
13. How is molecular spectroscopy used in police work?
14. What forces hold molecular substances in the liquid and solid states? Hint: this is the attraction between molecules, not the chemical bonds between atoms.
Unit 15, Kinetic Theory, Absolute Temperature, Atmospheric Pressure.
For research, go to The On-Line Reference Text Unit 15. Kinetic Theory
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
Hint for Probs 1-4: 1 kPa = 7.50 mm, so 1mm = 0.13 kPa. One atmosphere = 760 mm = 101 kPa.
For Problems 1,2,3, use Figure 15-3.

1. The open manometer in Figure 15-3 is filled with mercury and connected to a container of hydrogen. The mercury level is 80.0 mm (change to kPa) higher in the arm of the tube connected to the gas (so it's below atmospheric pressure). Atmospheric pressure is 99.0 kPa. What is the pressure of the hydrogen in kilopascals? Ans: 88.6 kPa.
2. A closed manometer like the one in Figure 15-3 is filled with mercury and connected to a container of nitrogen. The difference in the height of mercury in the two arms is 690 mm. What is the pressure of the nitrogen in kilopascals? Ans: 690mm = 90 kPa.
3. An open manometer is filled with mercury and connected to a container of oxygen. The level of mercury is 6.00 mm higher in the arm of the tube connected to the container of oxygen (so the oxygen is below atmospheric pressure). (Change mm into kPa). Atmospheric pressure is 100.0 kPa. What is the pressure, in kilopascals, of the oxygen? See Figure 15-3. Ans: 99.0 kPa.
Hint for Probs 5-8: Use the formula, K = C + 273, so C = K -273.
4. Convert the following temperatures from Celsius to Kelvin:
a. 87o,b. 16o , c. 59o , d. -68o , e. 73o. Hint: watch your signs!
5. Convert the following temperatures from Kelvin to Celsius:
a. 86o, b. 191o, c. 533o, d. 318o, e. 894o.
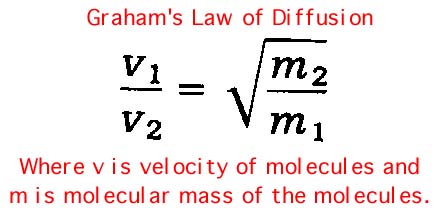
6. Suppose you have two vials, one containing ammonia and containing chlorine. When they are opened across the room which would you expect to smell first and why? Hint: Graham's Law of Diffusion relates velocity of molecules with molecular mass (heavier molecules move more slowly).
7. With regard to particle motion, what are the differences in the states of matter? Hint: Which moves fastest, solid, liquid, or gas molecules?
8. How does temperature affect the kinetic energy of a particle?
9. In terms of the kinetic theory, what is the significance of absolute zero?
10. What is an elastic collision? How does it differ from an inelastic collision? Hint: Molecules collide with elastic collision which means that no energy is lost. Bam, bam, biff, biff continues indefinitely. A pie in the face is inelastic.
11. What is the Kinetic Theory of Matter and list 9 evidences supporting the Kinetic Theory.
12. How did Torricelli discover atmospheric pressure?
13. How can we find the density of air and what is its value in g/L?
14. Give the values for one Atmosphere of pressure (sealevel average) in a) meters of water, b) millimeters of mercury, c) kilograms/cm2, and d) kilopascals.
15. Describe the demonstration of the Magdeburg Hemispheres and tell what they inform us.
16. What is the true meaning of suction?
For research, go to The On-Line Reference Text Unit 16. Solids
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
Hints for Ch 16 Probs: Basic Crystal Systems and Unit Cells in Action.
1. From the photo of the NaCl lattice, show why NaCl is the simplest formula.
2. Find the percentage of water in a crystal of CuSO4 • 5H20. Hint: compare the MM for five water molecules with that of one CuSO4 • 5H20 and get %. Ans: 36%
3. Use diamond and graphite to explain how bonding affects the properties of a crystal. See Graphite and Diamond and Buckyballs.
4. Cite reasons why nonmetallic elements have low melting points. Hint: Remember intermolecular forces (van der waals) vs. ionic forces.
5. How do the properties of a defective crystal differ from a perfect crystal?
6. How do amorphous substances differ from crystalline substances? Hint: Amorphous means without definite shape (like The Boom).
7. What are Bucky Balls?
8. Compare these terms: a) Isotope, b) Allotrope, c) Isomer.
9. Name the seven basic crystal structures.
10. Compare the structure of ice with that of liquid water and tell why there is a difference.
For research, go to The On-Line Reference Text Unit 17. Liquids
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
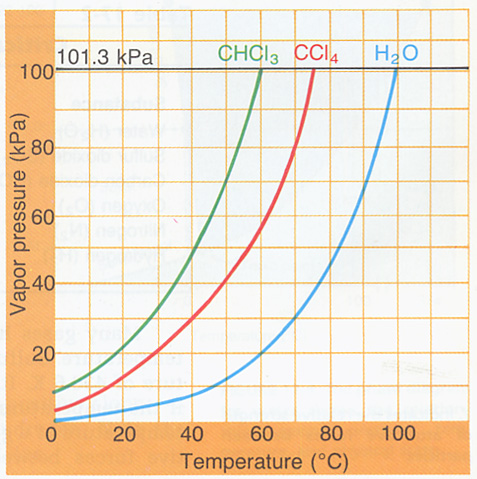
1. Using Figure 17-8 below, determine boiling points of the following under the conditions listed:
a. CHCl3 at 50.0 kPa, b. H2O at 100.0 kPa, c. CCl4 at 75.0 kPa, d. H2O at 11.0 kPa.
Figure 17-8
2. You have a sample of H2O with mass 23.0 g at a temperature of -46.OoC. How many joules of heat energy are necessary to:
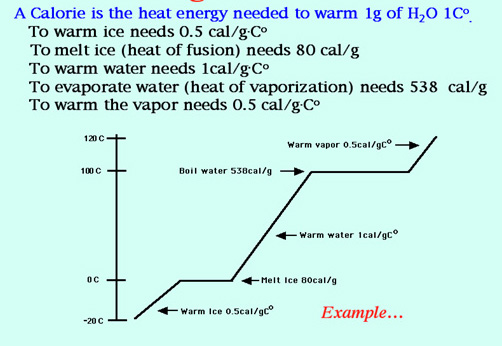
a. heat the ice to OoC?, b. melt the ice?, c. heat the water from OoC to 100oC?, d. boil the water?, e. heat the steam from 100oC to 109oC? The energies are as follows:
..... To warm ice (specific heat of ice) = 2.06 J/g•Co
..... To melt ice (the heat of fusion) = 334 J/g
..... To warm water (specific heat of water) = 4.18 J/g•Co
..... To evaporate water (heat of vaporization) = 2260 J/g
..... To warm water vapor (specific heat of steam) = 2.02 J/g•Co
.......... Ans: a = 2180 J, b = 7680 J, c = 96l0 J, d = 52000 J, e = 418 J.
3. Draw the Warming Curve for Water and label the FIVE parts.
4. Using the Warming Curve of Problem 3 and the Sample Problem found in your notes or at Sample Problem from the on-line notes, calculate the Total number of calories needed to change 20.0 grams of ice at -15.0oC to water vapor at +125.0oC. The Table of Heat Values for Water. Ans: 14,760 calories.
Table of Heat Values:
5. Define Boiling Point and describe two ways to boil a liquid.
6. What is critical temperature? What is critical pressure? Hint: Use the on-line text or your book.
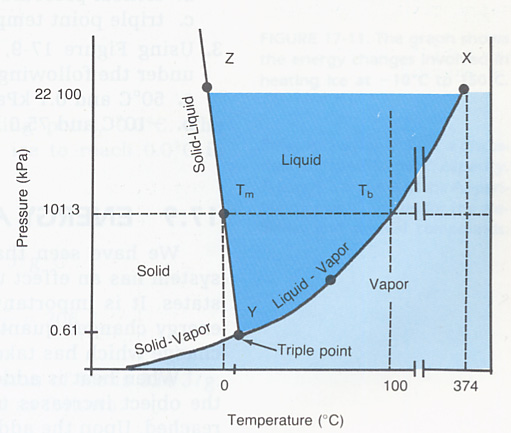
7. What is a triple point? Look at the phase diagram in Figure 17-9. determine the value of the triple point for water.
Figure 17-9. The Phase Diagram for water.
8. What is Le Chatelier's principle? How does pressing on a balloon demonstrate this principle?
9. What is sublimation? List three examples of a substance which sublimes.
10. Explain why the water cools when it is boiled with a vacuum pump.
11. Describe what happens to the crystal lattice as ice melts.
12. What is the difference between volatile and nonvolatile substances? Give a solid and a liquid example of each.
13. A well-stirred mixture of ice and water is at equilibrium. If a small amount of warm water or ice is added, the temperature doesn't change why?
For research, go to The On-Line Reference Text Unit 18. Gases
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
Partial Pressure Problems Hint: Use Dalton's Law of Partial Pressure
P(atm) = Pgas + P(water vapor), and Table 18-2.
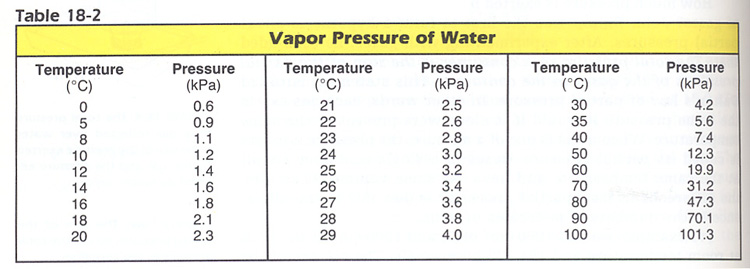
1. Find the partial pressure of H2 gas collected over water at 20oC when the atmospheric pressure is 98.0 kPa. Ans: 95.7 kPa.
Hint for the following problems. Use the Gas Law equation:
PV/T = P'V'/T'
Standard Pressure = 101 kPa
Standard Temperature = 273 K
2. Correct the volumes of the following gases as indicated: Remember that T must be in K. K = C + 273.
a. 7.51 L at 5.00oC and 59.9 kPa to STP. Ans: 4.36 L
b. 80.0 L at 35.0oC and 111 kPa to STP. Ans: 78.0 L
For Problem 3 you will need Graham's Law of Diffusion , The Rate of Diffusion is inversely proportional to the square root of the Molecular Masses. In other words, the heavy molecules move more slowly.

3. What is the ratio of the speed of helium atoms to the speed of radon atoms when both gases are at the same temperature? Ans: 7.45. The Periodic Table of the Elements.
4. Theoretically, what would happen to a gas which is cooled to absolute zero? Why can we not get colder than absolute zero?
For research, go to The On-Line Reference Text Unit 19. Gases and The Mole
Hint: The Ideal Gas Law Equation: PV = nRT. Where P is the pressure in kPa, V is the volume in L, n is the number of moles, T is the absolute temperature in K (K = C + 273), and R is the gas constant = 8.31 L•kPa/mol•K.
1. What pressure will be exerted by 0.300 mol of gas contained in an 8.00 L vessel at 18.0oC? Ans: 90.7 kPa. Remember to change oC into K.
2. How many moles of gas will occupy a 0.486 L flask at 10.0oC and 66.7 kPa pressure? Ans: 0.0138 mol. Hint: rearrange the equation, PV = nRT, solving for n.
3. What volume will be occupied by 0.362 mol of gas at 100.3 kPa and 8.00oC? Ans: 8.43 L. Hint: rearrange the equation, PV = nRT, solving for V.
4. At what temperature is a gas if 0.0851 mol of it are found in a 0.604 L vessel at 100.4 kPa? Ans: 85.3K = -187oC. Hint: rearrange the equation, PV = nRT, solving for T.
For the following you need a balanced equation. The Molar Volume for any gas at STP is 22.4 L/mol.
5. Balance the equation: Br2 + NaI ---> NaBr + I2. Find how many liters of Br2 gas are needed to procuce 5.0 grams of I2 at STP. MM of I2 is 254 g/mol. Ans: 0.44 L
For research, go to The On-Line Reference Text Unit 20. Energy and Disorder
Your Unit 20 assignment is to Read Unit 20 on Entropy. No written assignment.
Unit 21, Solutions, Unit 22, Colligative Properties
For research, go to The On-Line Reference Text Unit 21. Solutions and 22. Colligative Properties
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
The Periodic Table of the Elements
1. Calculate the molarity of the ion designated in the following solutions: Hint: you need a balanced ionic equation for dissolving to get the mole ratio of the ions. And M = mol/L. And mol = g/MM.
a. Br1- for 316 g MgBr2 in 859 mL solution. Ans: 4.00M.
b. Ca2+ for 8.28 g Ca(C5H902)2 in 414 mL of solution. Ans: 0.82M.
2. Calculate the molarity of the following solutions:
a. 31.1 g Al2(SO4)3 in 756 mL solution. Ans: 0.120M.
b. 59.5 g CaCl2, in 100 mL solution. Ans: 5.36M.
The Periodic Table of the Elements
3. Compute the masses of solute needed to make the following solutions:
Remember: g = (M)(MM)(L). L = mL/1000mL/L
a. 1.000 L of 0.780 M Sc(NO3)3. Ans: 180g.
b. 100 mL of 0.626 M VBr3. Ans: 18.2g.
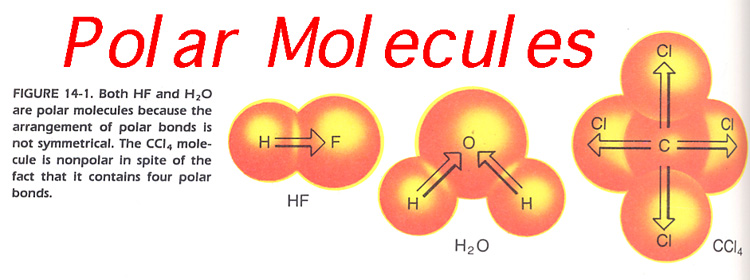
4. Iodine crystals are relatively insoluble in water, however they do dissolve in carbon tetrachloride. Explain why. Hint: Remember polar and non-polar molecules. Figure 14-1.
Figure 14-1
5. Reduce the following balanced equations to net ionic form. Hints: Ionize all aqueous substances (aq). Do not ionize solids (s) crystals (c) gases (g) or H2O(l). Then cancel out the identical ions on each side of the equation.
The Rules and Sample Problems:
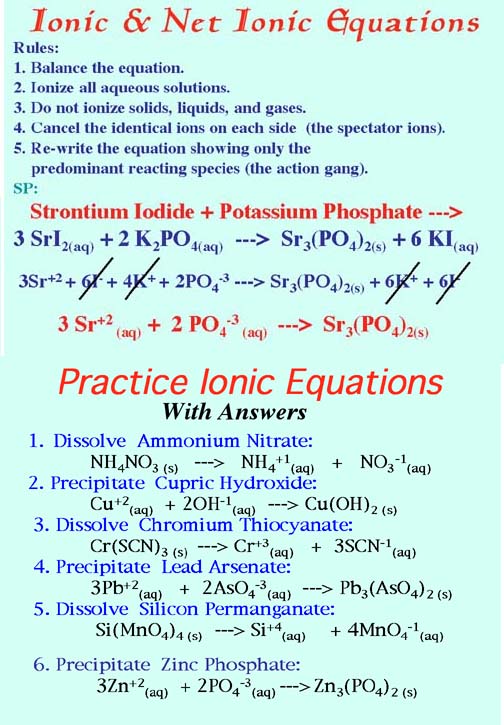
Show your work!
CoCl2(aq) + 2KOH(aq) ---> Co(OH)2(s) + 2 KCl(aq)
Ans: Co+2(aq) + 2OH-1(aq) ---> Co(OH)2(s)
3 Ag2CrO4(aq) + 2 AlBr3(aq) ---> Al2(CrO4)3(aq) + 6 AgBr(s)
Ans: 6 Ag+(aq) + 6 Br-(aq) ---> 6 AgBr(s) or Ag+(aq) + Br-(aq) ---> AgBr(s)
H2SO4(aq) + 2 NaOH(aq) ---> Na2SO4(aq) + 2 H2O(l)
Ans: 2 H+(aq) + 2 OH-(aq) ---> 2 H2O(l) or H+(aq) + OH-(aq) ---> H2O(l)
2 HCl(aq) + CaCO3(aq) ---> CO2(g) + H2O(l) + CaCl2(aq)
Ans: 2 H+(aq) + CO3-2(aq) ---> CO2(g) + H2O(l)
6. What are colligative properties? See Colligative Properties.
7. Define volatile and nonvolatile and give an example of each.
8. Define solute and give an example.
9. How does the addition of a solute cause the freezing point of the solvent to be depressed? Hint: the number of molecules or ions affect the colligative properties.
10. How does the addition of a solute cause the boiling point of the solvent to be elevated? Hint: the number of molecules or ions affect the colligative properties.
11. Define and explain the Tyndall Effect. Give three examples.
12. How does a colloid differ from a suspension? Give two examples. See Table 22-3.

13. What is Fractional Distillation? Give an example.
14. Would you expect water and butter to be miscible? Explain. Hint: Remember polar and non-polar molecules, See Figure 14-1 above.
15. What effect does a solute have on the boiling point of a solvent? See Colligative Properties above.
*** Here Endeth the First Semester ***
Hits on this page from 2000 to 2007 are 80,000.