Periodic Properties
![]()
Periodic Properties
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
PERIODIC PROPERTlES
We can see the periodic nature of the elements in groups as well as in periods of the table. The elements in the first group, hydrogen through francium all contain one s electron in the outer level.
A group is often called a family because of the similarity of the elements within it. Some families have a common name. We have already mentioned the alkali metal family and the halogen family.
The members of a family have a similar arrangement of outer electrons and thus tend to react similarly. For instance, lithium, sodium, and potassium all lose one electron to chlorine and form chlorides (LiC1, NaCL, KC1).
Hydrogen is also found in this group. It forms a chloride, hydrogen chloride. Let us take a closer look at some elements. From studying the properties of a few, we can predict the properties of many more.
11:1 HYDROGEN
Hydrogen, like the alkali metals, has only one outer electron. Because of its unique properties, it is usually considered, as a family by itself.
There are four ways in which the hydrogen atom can react.
It may lose its one electron to become a positive hydrogen ion. A positive hydrogen ion is simply a bare proton. Remember from Section 8:10 that a proton is about one trillionth the size of an atom. Because of its small size, the hydrogen ion has some unique characteristics. These characteristics will be considered in studying hydrogen bonding and acids later in this text.
The second way a hydrogen atom can react is by sharing its single outer electron. Most nonmetals react with hydrogen to form compounds involving shared electrons. Examples of these compounds are HCl and H2O. These compounds and their formation will be studied in more detail in Chapter 13.
The third way hydrogen can react is to gain an electron. When this change occurs, the atom becomes a hydride ion, H-. Such a reaction can take place only between hydrogen and atoms of elements which give up electrons easily.
The most reactive metals, Groups IA and IIA, form ionic hydrides. In these compounds, the radius of the hydride ion averages about 0.142 nm.
We can see that the hydride ion is larger than the fluoride ion. Such a radius indicates that the single proton of the hydride ion has a very weak hold on the two electrons. We would expect, then, that the ionic hydrides would not be highly stable. Experiment confirms this conclusion.
Ionic hydrides are found to be quite reactive compounds. By sharing or gaining electrons, hydrogen attains the stable outer level configuration of helium.
Hydrogen gas is used in the manufacture of other chemicals such as ammonia and methanol. One interesting use is the conversion of a vegetable oil such as corn oil into shortening, or oleo margarine. The reaction which takes place involves the addition (Chapter 30) of hydrogen atoms to double bonds (Chapter 13) in the oil molecules.
A fourth type of bonding involves the formation of bridges between two atoms by hydrogen atoms. The best examples of these compounds are found with the element boron and some of transition metals. A study of such compounds is beyond the scope of this book. Since they are not common compounds, their behavior is only a small fraction of the chemistry of hydrogen.
11.2 ALKALl METALS
The metals in Group IA are reactive. If one member of a family forms a compound with an element or ion, we can predict that other members will do the same. However, the members of a family are not the same in every way.
The outer electron of lithium occupies a volume closer to the nucleus than the outer electron of sodium. The sodium atom has many more electrons between the outer electron and the nucleus than lithium.
An increasing number of electrons between the outer level and the nucleus has a shielding effect. This effect blocks the attraction of the nucleus for the outer electrons. Larger atoms tend to lose their outer electrons more readily. This tendency is due to the distance of the electrons from the nucleus as well as the shielding effect.
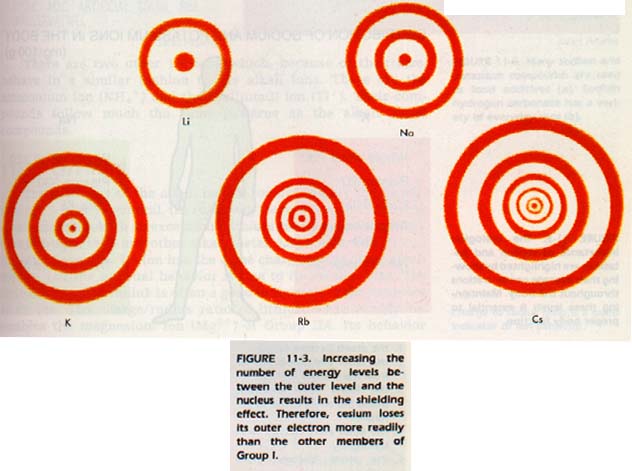
As the atoms of the alkali metals increase in size, the nucleus increases in positive charge. However, the force af the increased positive charge is more than offset by the outer electron's distance from the nucleus and the shielding effect. As a result, we find that the alkali metals lose their outer electrons more readily as we proceed down the group. This trend indicates that the most active metal would be francium, which is in the lower left corner of the periodic table.
Sodium and potassium ions are important biologically. The ratio of their concentrations in the body is vital to the transmission of nerve impulses.
Sodium compounds are among the most important in the chemical industry. Millions of tons of sodium hydroxide are consumed each year in producing other chemicals, paper, and petroleum products.
Sodium carbonate is also produced in millions of tons and used in manufacturing glass and other chemicals. Sodium sulfate is another substance used in manufacturing as well as paper and detergents.
Sodium silicate is widely as a catalyst in addition to being consumed in making soaps, paper, and pigments. A catalyst speeds up a reaction. tripolyphosphate is used as a food additive, softening water, and in making detergents.
There are two other 1+ ions which, because of their size, behave in a similar fashion to the alkali ions. These are the ammonium ion (NH4+) ion and thallium (T1+). Their compounds follow much the same patterns as the alkali metal compounds.
11:3 LlTHlUM
The reactions of the alkali metals involve mainly the formation of 1+ ions. In general, the reactivity increases with increasing atomic number, with an exception. Lithium reacts more vigorously with nitrogen than any other alkali metal.
Lithium is exceptional in other ways, too. Its ion has the same charge as the other alkali metals, but the unusual behavior is due to its smaller size. The ratio of charge to radius is often a good indication of the behavior of an ion. The charge/radius ratio of lithium more closely resembles the magnesium ion (Mg2+) of Group IIA. Its behavior resembles Mg2+ more closely than the next member of its own family, sodium, Na+.
This diagonal relationship is not unusual among the lighter elements. One example of this relationship is that lithium burns in air to form the oxide, Li2O, as does magnesium to form MgO.
The lithium atom also differs from the other alkali metal atoms in some physical properties. Unlike the other alkali metals which dissolve in each other in any proportions, lithium is insoluble in all but sodium. It will dissolve in sodium only above 380oC.
In other respects, lithium metal is like the other members of its family. For example, it is a soft, silvery metal with a low melting point, as are the other alkali metals except cesium which is yellow.
All of the alkali metals will dissolve in liquid ammonia to give faintly blue solutions. These solutions conduct electricity. The alkali metals form binary compounds with almost all nonmetals. In these compounds, nonmetals are in the form of negative ions. In solution, the lithium ion, because of its high charge/radius ratio, attracts water molecules more strongly than any other alkali ion.
11:4 ALKALlNE EARTH METALS
The alkaline earth metals are quite similar to the alkali metals except that they form the 2+ ion. Most of their compounds exist as ions and are soluble except for some hydroxides, carbonates, and sulfates. Beryllium is used in making nonsparking tools and magnesium is widely used in lightweight alloys. The other metals are too reactive to be used as free elements.
Two calcium compounds find large markets. Lime (calcium oxide) is used to make steel, cement, and heat-resistant bricks. It is also applied to soils which are too acidic to farm without treatment. Calcium chloride is used in a wide range of applications. One interesting use is in controlling road conditions through de-icing in the winter and keeping down dust in the summer.
11:5 ALUMlNlUM
Aluminum is the only metal of practical importance in Group IIIA. With three electrons in the outer level, it is less metallic than the elements of Groups IA and IIA. For instance, in forming compounds it tends to share electrons rather than form ions. It is also less reactive than Group IA and IIA metals. Large quantities of aluminum are consumed each year in producing lightweight alloys to make everything from soda cans to aircraft. Aluminum finds applications in water purification, paper manufacture, and fabric dyeing.
11:6 GROUP IVA
The elements of Group IVA have atoms with four electrons In the outer level. These elements generally react by sharing electrons. However, the tendency to lose electrons increases as atomic number of Group IVA elements increases.
There are a few compounds in which carbon in the form of a carbide ion, C4-, be considered to exist. Silicon, the next member of this family, although nonmetallic, does not form 4- ions under any condition. However, there are compounds in which silicon exists as 4+ ions.
The major part of carbon chemistry is classed as organic chemistry. Carbon itself, carbonic acid and its salts, carbidies, cyanides, and the oxides and sulfides of carbon are considered inorganic.
Most organic compounds involve sharing electrons between a carbon atom and one or more other carbon atoms. The tendency to form "chains" of similar atoms is called catenation. Only carbon exhibits catenation to any great extent.
Carbon is found in nature in three different molecular forms, diamond, graphite, and fullerines.
In diamond, each carbon atom shares electrons with the four nearest carbon atoms.
In graphite, the sharing is to the nearest three carbon atoms.
In fullerines (buckeyballs), a cage of carbon atoms is formed (like a soccer ball).
The difference in structure results in different properties for these three forms of carbon. We will study these differences in Chapter 16.
Different forms of the same element are called allotropes.
Industrially, carbon is used in a form called "carbon black" made by burning natural gas or other fuel. Carbon black is often referred to as "soot" when it has accumulated in a place where it is unwanted. It is actually a microcrystalline form of graphite, and is used as a black pigment and wear-resistant additive in rubber tires.
Carbon dioxide gas is a byproduct of several chemical processes. It is collected, compressed, and sold as a liquid in steel cylinders. Customers use it for refrigeration, carbonating beverages, and producing other chemicals.
Silicon shows only a slight tendency toward catenation, much less so than carbon. However, the chemistry of silicon, like that of carbon, is characterized by electron sharing.
Silicon is the second most plentiful element in the earth's crust. (Oxygen most plentiful.) It is found in a large number of minerals and is bound to oxygen atoms in a variety of ways. In these compounds, called silicates, each silicon atom is surrounded by four oxygen atoms with which it shares electrons. Transistors, chips, and synthetic motor oils are three developments of silicon chemistry. Carbon and silicon differ more than any other two vertically neighboring elements in the periodic table.
Tin and lead are distinctly metallic members of Group IVA. They are quite similar except that for tin the 4+ state is more stable than the 2+ while the reverse is true for lead.
These two metals are easily produced from their ores and have both been known since early times. In general, their uses are based on their lack of chemical reactivity. They are common components of alloys which are mixtures of metals. Examples of alloys are solder, which contains lead and tin, and bronze, which contains copper and tin.
11:7 NlTROGEN AND PHOSPHORUS
Nitrogen and phosphorus differ quite a good deal for adjacent members of the same family. Nitrogen occurs in all oxidation states ranging from 3- through 5+. Phosphorus shows only 3-, 0, 3+, and 5+. Liquid nitrogen is used to maintain very low temperatures.
The unreactive gas is used to surround reactive materials which would otherwise react with oxygen in the air. Elemental nitrogen, N2, gas, is one of the most stable substances known.
Most nitrogen compounds are relatively unstable, tending to decompose to N2. Conventional high explosives, for example, trinitrotoluene (TNT) and dynamite, utilize nitrogen compounds. Nitrogen compounds are produced naturally from atmospheric nitrogen by nitrogen-fixing bacteria.
These bacteria convert molecular nitrogen to a form which can be used readily by plants. This process is essential to plant life. Nitrogen compounds a vital function in living systems in amino acids, essential components of proteins.
Nitrogen compounds are important to living systems.
However, most nitrogen compounds are produced from atmospheric nitrogen by synthetic processes. Huge quantities ot liquid N2 are obtained from the air in the Haber Process.
The most common use for ammonia is as a fertilizer. Much of the ammonia not used directly as a fertilizer is converted to other nitrogen compounds which are themselves fertilizers.
An example is ammonium nitrate, which is also used in explosives. Some ammonia is converted to nitric acid. Nitric acid itself is widely used in the manufacture of fertilizer and explosives. Large quantities of ammonium sulfate are obtained from the steel industry's conversion of coal to coke.
Elemental phosphorus occurs as P4 molecules and is solid at room temperature. The P4 molecules "stack" in different ways to form several allotropes.
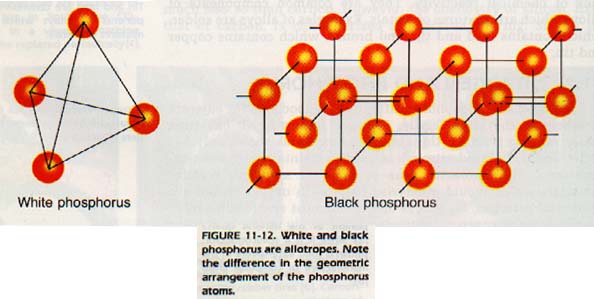
One allotrope, white phosphorus, is so reactive that it ignites spontaneously on contacting air.
Red must be exposed to a flame to ignite. The principal source for phosphorus in nature is phosphate rock, Ca3(P04)2, Phosphate rock is used in producing Ca(H2P04)2 and for use as fertilizer.
Some phosphate rock is converted to phosphoric acid which is also used primarily in making fertilizer but has other applications in industry.
Organic compounds of phosphorus are vital to living organisms. Utilization of energy by living systems involves a compound adenosine triphosphate (ATP). The transfer of genetic information from generation to generation involves deoxyriboacid (DNA). Each DNA molecule contains hundreds of groups. Ribonucleic acid (RNA), used by cells in also contains phosphate groups.
11:8 OXYGEN
Oxygen is the most plentiful element in the earth's crust. It combines with all other elements except helium, neon, and argon. Since the atom has six electrons in its outer level, it can gain two electrons to achieve the stable octet configuration of neon. In so doing, it becomes the oxide ion, 02-.
It can also react by sharing electrons. With metals which tend to lose electrons readily, oxygen forms ionic oxides. With nonmetals, oxygen tends to share electrons. The behavior of these oxides when dissolved in water depends on their structure.
Ionic oxides generally react with water to produce basic solutions. However, oxides formed by the sharing of electrons tend to react with water to form acidic solutions.
There are some oxides which can produce either acidic or basic solutions, depending on the other substances present. Such oxides are called amphoteric oxides.
Like carbon, oxygen has allotropes. Oxygen usually occurs in the form of diatomic oxygen molecules, 02, The free oxygen you breathe from air is 02.
There is another allotrope of oxygen called ozone. Ozone is the triatomic form of oxygen, O3 , and is highly reactive.
Ozone can be synthesized by subjecting 02 to an electric discharge. It is formed naturally in small amounts by lightning, and in the upper atmosphere by ultraviolet radiation from the sun.
That layer of ozone protects living things on the earth from the sun's harmful ultraviolet radiation. In Europe, ozone is the main chemical used in water purification.
Pure oxygen is extracted from air, compressed, and sold in cylinders. Its largest uses are the production of steel, artificial breathing atmospheres, rocket engines, and welding torches.
The chemistry of sulfur is similar to the chemistry of oxygen, especially in the behavior of the 2- ion. The S2- ion shows similar characteristics to oxygen in solubility and acid-base behavior.
Unlike oxygen, whose longest chain is O2, sulfur can form long chains of atoms attached to the S2- ion. These ions are called polysulfide ions. Sulfur exhibits two important positive oxidation states represented by the oxides S02 and SO3.
When sulfur or sulfur compounds are burned in an ample supply of air, SO2 is produced.
Using a catalyst, SO2 can be converted to S03. When SO3 is dissolved in water, sulfurous acid (H2SO3) is produced.
If S03 is combined with water, sulfuric acid (H2SO4) is produced. The combination of SO3 and water is done by dissolving the SO3 in H2S04 and then adding water.
Sulfuric acid is produced in huge quantities. Twice as much H2S04 is produced as the next most common chemical. The principal sulfuric acid-consuming industries in the United States are fertilizer, petroleum refining, steel, paints, and pigments.
11:9 HALOGENS
Group VIIA contains fluorine, chlorine, bromine, iodine, and astatine. The elements of this group are called the halogen (salt forming) family.
In many chemical reactions, halogen atoms gain one electron. They become negatively charged ions with a stable outer level of eight electrons.
As in other families already discussed, three factors determine the reactivity of the halogens. They are the distance between the nucleus and the outer electrons, the shielding effect of inner level electrons, and the size of the positive charge on the nucleus.
Fluorine atoms contain fewer inner level electrons than the other halogens, so the shielding effect is the least. The distance between the fluorine nucleus and its outer electrons is less than the other halogens. Thus, the fluorine atom has the greatest tendency to attract other electrons.
This attraction makes fluorine the most reactive nonmetal. The astatine nucleus has the largest number of protons and the largest positive charge. However, the increased charge on the nucleus is not enough to offset the distance and shielding effects. Thus, of all halogen atoms, the astatine nucleus has the least attraction for outer electrons.
Notice that fluorine is active because the atoms of fluorine have a great tendency to gain one electron and become negative ions.
The active metals are active because they hold the single outer electron loosely and it is easily removed.
The groups between IA and VIIA vary between these two extremes. In general, on the right side of the table, the nonmetallic elements become more active as we move from the bottom to the top.
On the lefthand side of the table, the metals become more active as we move from the top to the bottom. The most active elements are located in the upper right-hand and lower left-hand corners of the periodic table.
11:10 FLUORlNE AND CHLORlNE
The halogens are the most reactive nonmetallic family. They usually react by forming negative ions or sharing electrons. Fluorine is the most reactive of all the chemical elements. It reacts with all other elements except helium, neon, and argon.
Fluorine is obtained from the mineral fluorspar, CaF2. Like hydrogen, halogen atoms can form bridges between two other atoms. An example of such a compound is BeF2. The halogens also form a large number of compounds among themselves. Examples are CIF, CIF5, BrF5, IF5, IF7, BrCI, and ICI3.
Chlorine, though less abundant than fluorine in the earth's crust, is more commonly found in both the laboratory and industry. Chlorides of most elements are available commercially.
These compounds are quite often used in the laboratory as source of positive metal ions bound with the chloride ions. Chlorine is produced primarily to make other chemicals, some is consumed in paper manufacture. Hydrochloric acid is a byproduct of many industrial processes. Its uses include manufacture, dye production, food processing, and oil drilling.
The commercial preparation of chlorine led to one of major water pollution crises of the early 1970s. The chlorine produced by running an electric current through a solution sodium chloride in water. One of the substances used to conduct current in the apparatus was mercury. The leakage of mercury into nearby water sources caused a public outcry.
Industries had to remove the mercury from their waste before dumping, or go to another method of producing chlorine. Chemists solved problem by designing new cells. There are many other pollution problems which must be given special attention by chemists now.
radon
11:11 NOBLE GASES
For many years after their discovery Group VIIIA, the noble gases, were believed to be chemically unreactive or inert. However, in 1962, the first "inert" gas compound was synthesized. Since these gases are not inert, we will refer to them as the noble gases. The first compound made was xenon hexafluoroplatinate (XePtF6).
Other compounds of xenon were soon produced. Once the techniques were known, the compounds could be made with increasing ease. Xenon difluoride (XeF2) was first made by combining xenon and oxygen difluoride (OF2) in a nickel tube at 300oC under pressure. The same compound can now be made from xenon and fluorine. An evacuated glass container of fluorine and xenon is exposed to daylight. The equation can be written as
Xe(g) + F2(g) ---> XeF2(c)
Compounds of xenon with oxygen and nitrogen have also been synthesized. Krypton and radon compounds have also been produced. Argon and helium are used to protect active metals during welding. Aluminum, for example must be welded in an argon or helium atmosphere. Argon is also widely used to fill light bulbs. Neon, krypton, and xenon are used to fill brightly colored gas discharge tubes for advertising.
111:12 TRANSITION METALS
The transition metals ("B" groups) are those elements who highest energy electrons are in the d sublevels. In all groups except IIB, the d orbitals are only partially filled. These partially filled d orbitals make the chemical properties of the transition metals different from the "A" group metals.
The major uses of the transition metals are as structural elements. The fourth period elements, titanium through zinc, are our principal structural metals. These metals are used either alone or as alloys. The only important transition metal compound produced commercially is titanium(IV) oxide, TiO2. The TiO2 is used extensively as a white paint pigment.
11:13 CHROMlUM
Chromium exhibits the typical properties of a transition metal. One property of chromium which is industrially important is its resistance to corrosion. Large quantities are used to make stainless steel and to "chromeplate" regular steel. In both cases, the chromium protects the iron in steel from corrosion.
Chromium usually reacts by losing three electrons to form the Cr3+ ion. Also of importance is the 6+ oxidation state. This state is formed when chromium loses all five of its 3d electrons, as well as its outer level 4s electron.
Two polyatomic ions are important examples of the 6+ state. These ions are the chromate ion, Cr042- and the dichromate ion, Cr2072-. Chromium also forms a Cr2+ ion. However, this ion is easily converted by oxygen in air to Cr3+. Consequently, the 2+ state is not of great importance. The 4+ and 5+ states are so unstable, they are just laboratory curiosities.
11:14 ZlNC
Although zinc is classed as a transition element, its behavior differs slightly due to a full d sublevel. It exhibits only one oxidation state, 2+. The special stability of the full d sublevel leaves only the two outer 4s electrons available for reacting. Zinc is the second most important transition element (after iron) in biological systems. Over 25 zinc-containing proteins have been discovered. As an example, lack of zinc in the diet prevents the pancreas from producing some digestive enzymes.
Metallic zinc, like chromium, is corrosion resistant. It is used extensively as a coating to protect iron. The coating can be applied in three ways. When the iron is dipped in molten zinc, the process is called galvanizing. The coating is also applied electrically. The third method is to allow gaseous zinc to condense on the surface of iron. Another major use for metallic zinc is in the production of alloys. Especially important is its combination with copper to form brass
11:15 NEODYMlUM
The electron configuration of neodymium (knee o DIM ee um) ends 6s24f4. From that configuration we would predict a 2+ oxidation state. However, Nd, as all the lanthanides, shows 3+ as its most stable state. The 3+ ion is pale violet in solution. The metal itself is soft and quite reactive. It tarnishes when exposed to air and has little practical use.
The compound Nd203, is used in glass filters and in some lasers. Neodymium is the second most abundant lanthanide metal. Its compounds are separated from the other lanthanides by a chromatographic technique (Chapter 14). The metal is obtained from the fluoride by a single displacement reaction.
3Ca + 2NdF3 ---> 3CaF2 + 2Nd
11:16 CURlUM
Curium's predicted electron configuration ends 7s25f8. However, its actual configuration is 7s25f76dl. Plainly, the stability of the half-full f sublevel more than offsets the promotion of one electron to the 6d. The element exhibits a 3+ oxidation state in its compounds, and the ion is pale yellow in solution.
The element itself is a silvery, hard metal of medium density and high melting point. It does not occur in nature. All curium (multigram quantities) has been produced by the slow neutron bombardment of the artificial element plutonium. It is reactive and highly toxic to the human organism. This metal is used as the energy source in nuclear generators in satellites.
SUMMARY
1. The most active metals are listed toward the lower left-hand corner of the table. The most active nonmetals are listed toward the upper right-hand corner.
2. Hydrogen is often considered as a family by itself because of the unique ways in which it reacts.
3. The alkali metals (Group IA) are the most active metals and react by losing one electron to form 1+ ions.
4. The alkaline earth metals (Group IIA) are second only to alkali metals in terms of reactivity. They form 2+ ions.
5. Aluminum (Group IIIA) is less reactive than the alkaline earth metals and forms 3+ ions.
6. In Group IVA, carbon and silicon have four electrons in the outer level and react by sharing electrons. Tin and lead, in the same family, are distinctly metallic.
7. Nitrogen and phosphorus (Group VA) form compounds by sharing electrons, but differ from each other considerably.
8. In Group VIA, oxygen is distinctly nonmetallic and reacts by gaining two electrons or by sharing electrons. Sulfur is similar to oxygen but also exhibits positive oxidation states.
4. The halogens (Group VIIA) are the most active nonmetallic elements and usually react by gaining one electron.
10. The noble gases (Group VIIIA) have very stable outer electron configurations and are much less reactive than most of the other elements.
11. Chromium is a typical, corrosion resistant transition metal exhibiting more than one oxidation number.
12. Zinc is corrosion resistant. However, because of its full d sublevel, it does not exhibit more than one oxidation state as do most transition metals.
13. Lanthanides exhibit the 3+ oxidation state. Curium, an actinide, also exhibits the 3+ oxidation state.
More on the Periodic Table:
For a PowerPoint presentation Click Here.
.......... The Song of The Elements by Tom Lehrer
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page