with hints and answers to problems
Second Semester
Units 23-29
with hints and answers to problems
Second Semester
Units 23-29
These are the problems for your assignments. Please do them for each Unit assignment.
Important Charts and Tables
Quick Unit Finder
Do not click here until the page is completely loaded!!!
***Here Beginneth The Unit Assignments: ***
Questions must be answered in COMPLETE SENTENCES, and the METHOD OF SOLUTION must be shown for the Calculations (the Hup, Two, Three, Four)!
Assignments must be headed properly!
All assignments MUST be done IN INK!
Assignments will not be graded if the above is not done.
It is best to do these assignments ON-LINE, because they have hot links to charts, tables, problems, and hints.
For research, go to The On-Line Reference Text or use your Text Book.
They must be done in INK and the Method of Solution must be shown for the Calculations!
For research, go to The On-Line Reference Text Unit 23. Reaction Rates
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
Hints: Remember [ ] means concentration, M, in mol/L. And L = ml/1000 ml/L.
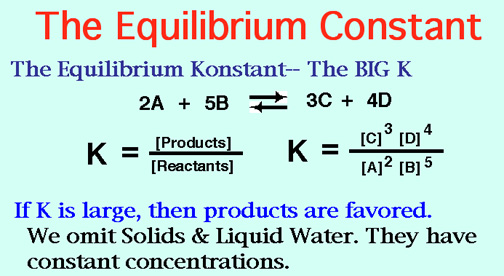
Also The Equilibrium Constant Formula. And The Rate Law.
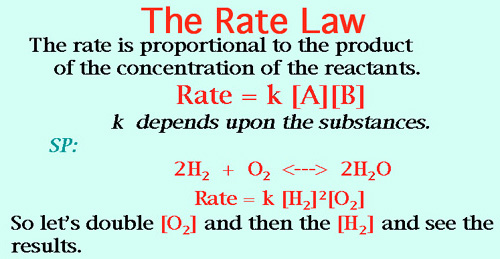
Ah, well let's double the O2, (2)1 = double rate. Now we'll double the H2, (2)2 = four times the rate. So (double) time (four times) gives us eight times the rate for the reaction! WOW!
1. Assume that NO(g) and H2(g) react according to the rate law: rate = k[N0]2[H2]. How does the rate change if
a. the concentration of H2 is doubled? Ans: doubled (explain).
b. the volume of the enclosing vessel is suddenly halved? Hint: Halving the volume doubles the concentration of both reactants. Ans: 8 times (explain).
c. the temperature is decreased? Ans: slows down (explain).
2. At a given temperature, the K(eq) for the gas phase reaction,
2HI(g) <----> H2(g) + I2(g) , is 1.40 x 10-2. If the concentrations both H2 and I2 at equilibrium are 2.00 x 10-4 M, find [HI]. Ans: = l.69 X 10-3.
3. At a given temperature, the reaction (all gases) CO + H2O <----> H2 + CO2 produces the following concentrations:
CO = 0.200 M, H2O = 0.500 M, H2 = 0.32M, CO2 = 0.42 M. Find the K(eq) at that temperature. Ans: 1.34.
4. Hydrogen sulfide decomposes according to the equation:
2H2S(g) <----> 2H2(g) + S2(g). At 1065oC, measurement of an equilibrium mixture of these three gases shows the following concentrations:
H2S = 7.06 x 10-3 M, H2 = 2.22 X 10-3M, and S2, = 1.11 x 10-3 M. What is the value of K(eq) for this equation? Ans: 1.10 X X10-4.
5. At 60.2oC, the equilibrium constant for the reaction, N204(g) <----> 2N02(g), is 8.75 x 10-2. At this temperature, a vessel contains N204 at a concentration of 1.72 x 10-2 M at equilibrium. What concentration of NO2 does it contain? Ans: 3.88 X 1O-2 M.
6. Explain the Collision Theory of reactions.
7. What are the five factors determining the rate of a reaction?
8. What are the two factors of Activation Energy?
9. What are the three factors needed to have a fire.
10. What is Spontaneous Combustion?
11. Draw an Activation Energy Curve for an exothermic reaction, label the parts and explain it.
12. Draw an Activation Energy Curve for an endothermic reaction, label the parts and explain it.
Unit 24, Acids, Bases, Salts and
Unit 25, Solubility Product and pH
For research, go to The On-Line Reference Text Unit 24. Acids, Bases & Salts
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
1. When NH4N03 is dissolved in water, the solution gets cold. Is the heat of solution of NH4N03 in water positive or negative? Hint: Think endo- or exothermic.
2. Give the name and formula of the salts obtained from complete neutralization reactions between the following acid-base pairs. Hint: Acid + Base ---> a Salt + Water. And Hydroxides are bases and Hydrogen compounds are acids.
a. sodium hydroxide and phosphoric acid (H3PO4)
b. potassium hydroxide and boric acid (H3B03)
c. chromium(III) hydroxide [Cr(OH)3] and perchloric acid (HClO4)
d. cadmium hydroxide and hydrobromic acid (HBr)
e. lithium hydroxide and silicic acid (H4Si04)
3. How does a Lewis acid differ from an Arrhenius acid?
4. Would you expect methane gas to dissolve in water? Hint: Consider polar and non-polar molecules. Methane is CH4, tetrahedral bonding. Water is p-2 bonding, Mickey Mouse ears.
5. Differentiate among unsaturated, saturated, and supersaturated solutions. Hint: Ah, Mr. Creosote.
6. What is the difference between concentrated and dilute solutions?
For research, go to The On-Line Reference Text Unit 25. Solubility Product/pH
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
Hints: Sample Solubility Product problem, and The Solubility Product Table:
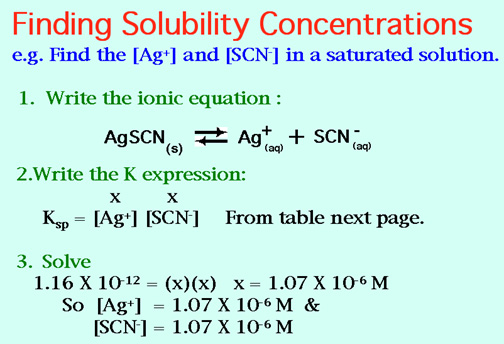
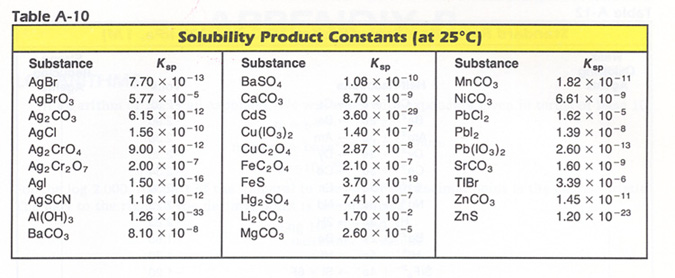
7. The solubility product constant of silver iodide is 1.50 x 10-16. What is [Ag+] in a solution at equilibrium? Ans: 1.22 X 10-8M.
8. If [D+] is 2.00 x 10-5 M at equilibrium, what is the Ksp for D2A ? Ans: 4.00 X l0-15 M.
9. What is the concentration of Ba2+ in a saturated solution BaCO3 ?
Ksp = 8.10 x 10-8. Ans: 2.85 X 10-4 M.
Hints: For water, Kw = [H3O+][OH-] = 1.00 x 10-14.
10. What is the hydroxide ion concentration in a solution with hydronium ion (aqueous hydrogen ion) concentrations 6.80 x 10-10 M ? Ans: 1.47 X 10-12 M.
11. What is the hydronium ion concentration in a solution with hydroxide ion concentration 5.21 x 10-3 M ? Answer: 1.9 x 10-12 M.
Hint: The pH System. For pH problems, Round Off (rough it) so you won't have to do logarithms.
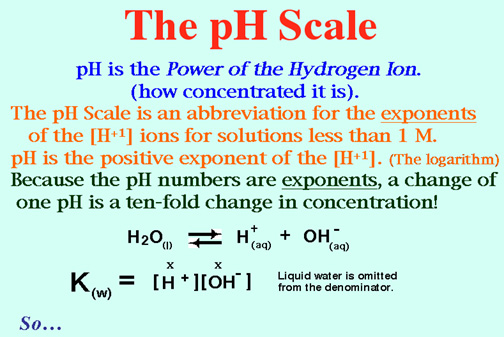
12. Find the pH of solutions with: the following H30+(aq) concentrations:
a. 1.0 x 10-3 M, b. 1.OO x lO-6 M, c. 1.0 x 1O-10 M, d. 1.0 x 10-6 M, e. 1.0 x 10-8 M, f.1.0 x 10-14 M. Ans: a = 3, b = 6, c = 10, d = 6, e = 8, f = 14.
13. Find the H30+(aq) concentration of the following solutions:
a. pH = 8, b. pH = 4, c. pH 8, d. pH = 10, e. pH 1.
Ans: a = 1 X 10-8 M, b = 1 X 10-4 M, c = 1 X 10-8 M, d = 1 X 10-10 M, e = 1 X 10-1 M.
Hint: Acid Base Neutralization and Titration.
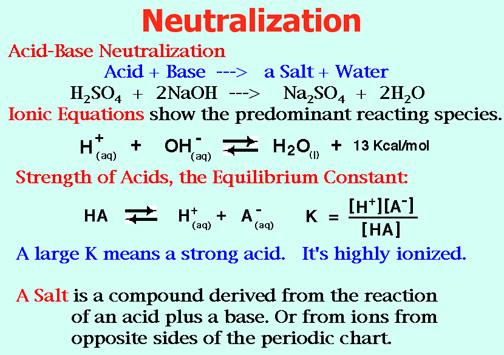

Remember: at the End Point:
Mol of Acid = Mol of Base, so
ML(acid) = ML (base) , and
L = mL/1000mL/L
14. What volume in mL of 0.196M LiOH is required to neutralize 27.3 mL of 0.413M HBr in
HBr + LiOH ---> LiBr + H20, so
the net ionic equation is
H+ + OH- ---> H2O
Ans: 57.5 mL
15. What is the value for the ion product constant (big K) for water, Kw?
16. What is the pH of 0.0001M NaOH? Hint: Don't confuse pH with pOH! And remember that K = [H+] [OH-]. And Kw = 1 X 10-14. Ans: pH=10.
17. What is the molarity of 1.00 liter of a solution containing 46.6 g Hg(CN)2? Hint: M = mol/L, and mol = g/MM. Ans: 0.184 M.
Unit 26, REDOX, Unit 27, Electrochemistry
For research, go to The On-Line Reference Text Unit 26. Oxidation-Reduction and 27. Electrochemistry
1. Define: Oxidation Number, Valence, Ion, Electrolyte, Electrode, Electrochemical Cell, Battery, Oxidation, Reduction, REDOX.
2. In the following, give the oxidation number for the indicated atoms: Hint: Write x, the oxidation number, over the element and add up the oxidation numbers of the known elements. See Rules of Oxidation Numbers.
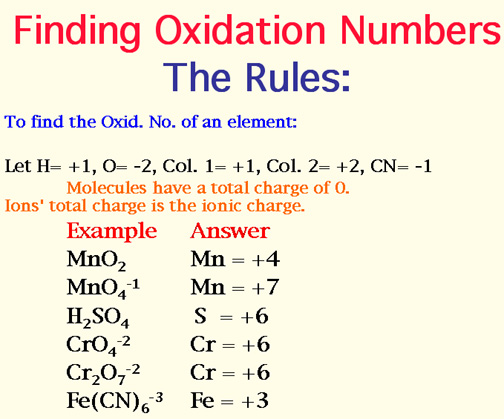
a. S in Na2S03 , b. Mn in KMn04, c. N in Ca(NO3)2, d. C in Na2C03, e. N in NO2, f. S in HSO4-, g. S in H2S207, h. S in A12S3 (let Al = +3), i. Mn in MnCl2 (let Cl = -1), j, C in C12H22011. Ans: Show work!
Click here for Appendix 8 Half-Reactions Chart.pdf. Then do the following three half-reaction equations, find the total voltage, and predict if they will go:
3. Mg + Al+3 ----->
4. SO4-2 + Co ----->
5. NO2(g) + Cr+3 ----->
For the in-class Appendix 8 Problems to be attached to this assignment, Click Here.
See Rules for balancing by oxidation numbers (ups and downs). And do the following three equations:

6. MnO2-1 + Sn+2 + H+ -----> Mn+2 + Sn+4 + H2O
7. AsO4-3 + NO + H+1 -----> NO3-1 + As2O3 + H2O
8. Cl-1 + NO3-1 + H+ -----> ClO-1 + NO + H2O
For the in-class Oxidation Numbers (ups & downs) problems to be attached to this assignment, Click Here.
9. What does an oxidizing agent do ? Hint: LEO, GER!
10. Draw an electrochemical cell and label the parts.
Remember that extra help is found in the Text book, the on-line Research Text, and in Extra Help on our web page.
For research, go to The On-Line Reference Text Unit 28. Nuclear Chemistry
Define these terms and give an example:
1. Proton, 2. Electron, 3. Neutron, 4. Beta particle, 5. Alpha particle, 6. Gamma ray, 7. Atomic number, 8. Mass number, 9. Isotope,10. Atomic weight.
Complete these nuclear reactions. Be sure that they are balanced in mass and charge:
11. 94Pu239 + 0n1 ---> ?
12. 45Rh107 ---> 46Pd107 + ?
13. 6C12 + ? ---> 102No254 + 2 0n1
14. 2He4 + 13Al27 ---> 14Si30 + ?
15. 92U238 + ? ---> 92U239
16. 1H2 + 1H2 ---> ? + 0n1
17. ? + 0n1 ---> 94Pu241
18. 3Li6 + 0n1 ---> ? + 1H3
19. 99Es254 + 2He4 ---> ? + 2 0n1
20. 5B11 + 94Cf 251 ---> ? + 3 0n1
Answers: 11. 94Pu240, 12. -1e0, 13. 96Cm244, 14. 1H1, 15. 0n1, 16. 2He3, 17. 94Pu240, 18. 2He4, 19. 101Md256, 20. 99Es259.
For research, go to Organic Chemistry
Cover the red answers, write your answers, then check yourself.
Problems 1 & 3 (no 2):
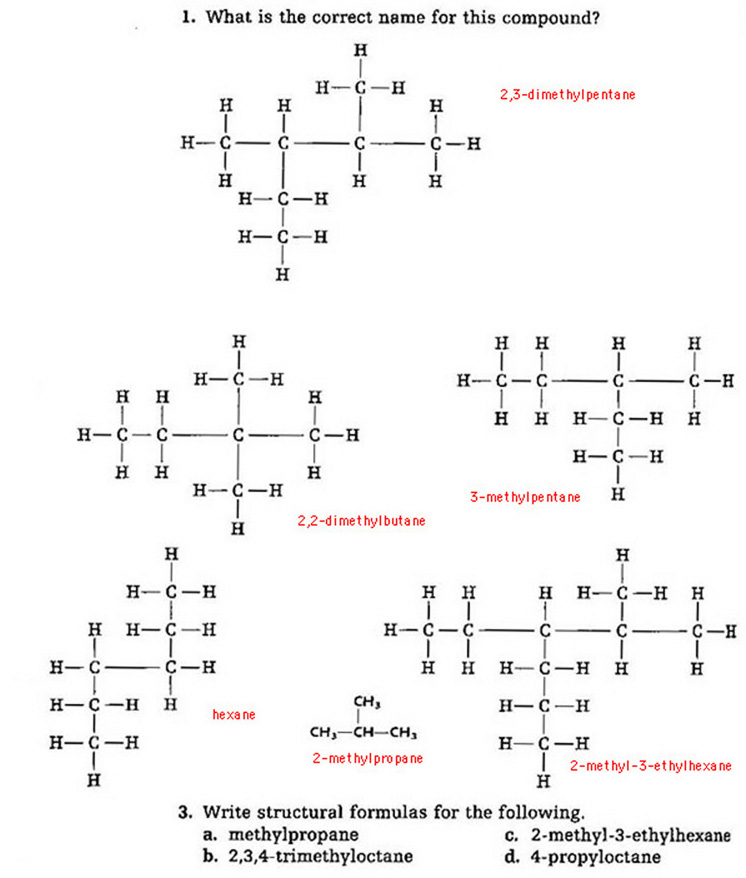
Problems 4-7:
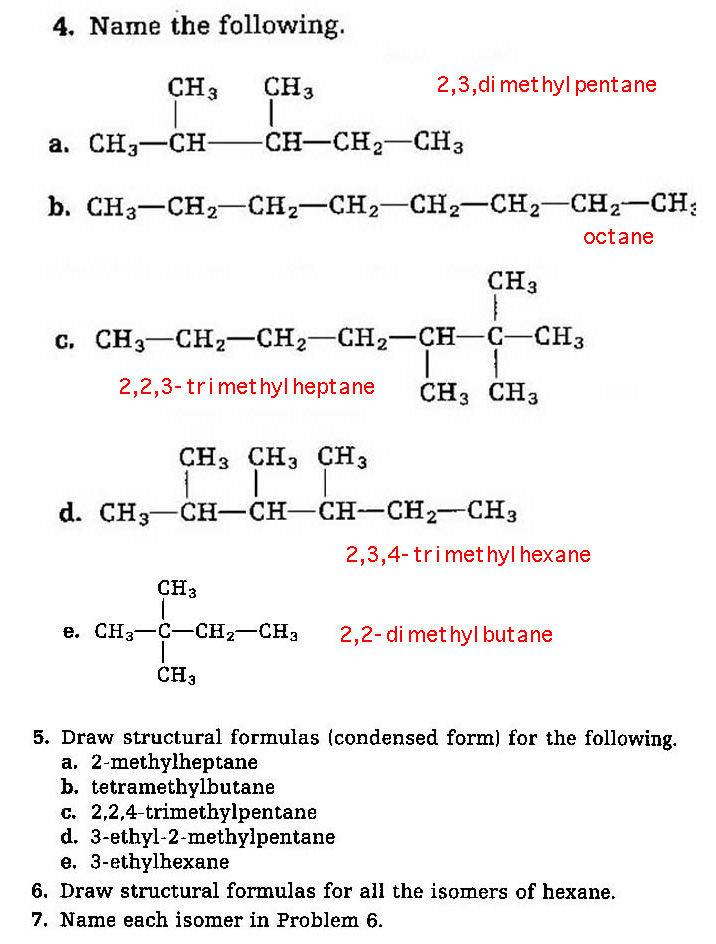
8. What is a homologous series?
9. Write the general formula for alkenes.
10. Write a formula for:
...... a. a 6 carbon alkene, b. an 8 carbon alkane, c. a 10 carbon alkyne.
11. What reasons can you cite as to why the boiling point of alkanes increases as a function of their molecular mass? Hint: Boiling points are caused by intermolecular (van der Waals) forces.
12. Write structural formulas for octane and nonane.
13. Which of the following compounds octane, pentene, heptyne would you predict to be most reactive? Why?
14. Write the formula for TNT, 2,4,6-trinitrotoluene.
15.Write the general formula for an alkyne.
No Numbers 16-22.
Problems 23-25, 29, (no 26, 27, 28):
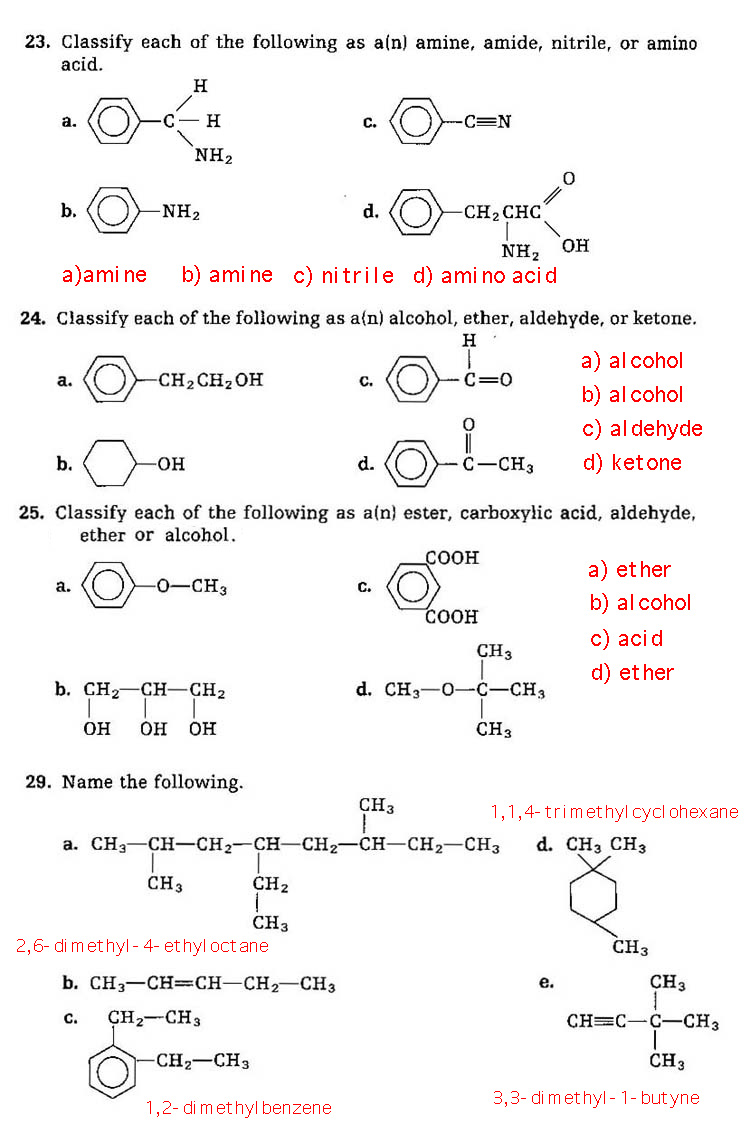
Problems 30, 32, 33 (no 31).
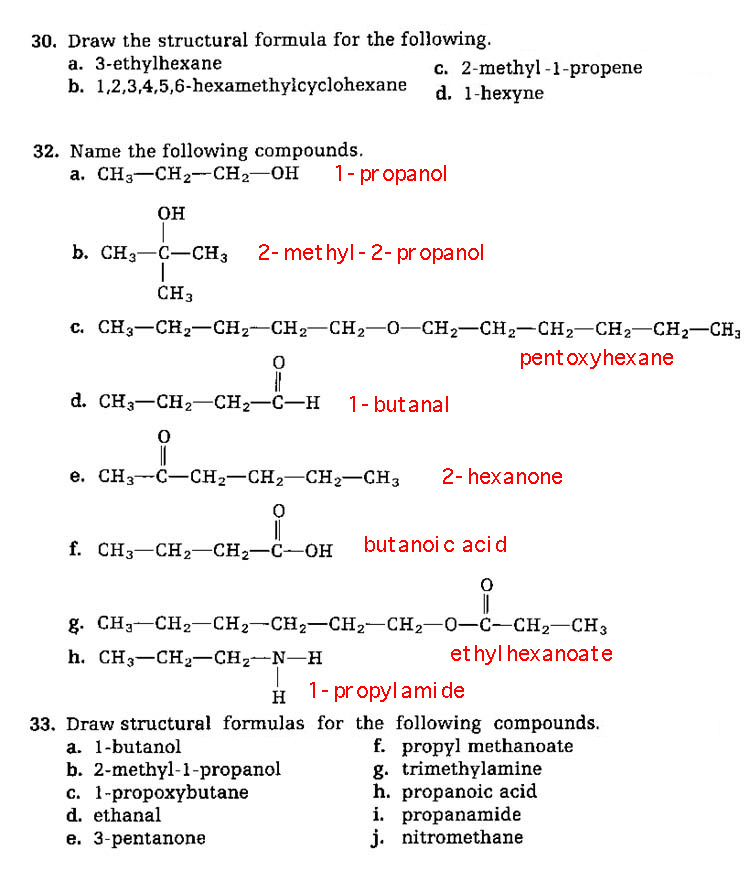
*** Here Endeth the Second Semester ***
Hits on this page from 2000 to 2007 are 300,000.