Acids, Bases, and Salts
![]()
Acids, Bases, and Salts
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
ACIDS, BASES, AND SALTS
Many foods have distinctive tastes. Some are bitter. Other foods are sour or salty. It is now known that a lemon or a grapefruit has a sour taste because it contains a compound called an acid. Soaps that contain lye, a base, taste bitter. Salty foods taste salty because they contain a salt, sodium chloride. The presence of these compounds, called acids, bases, and salts, gives many of our foods their distinctive flavors.
It was discovered long ago that these substances, when dissolved in water, conduct an electric current. Because they conduct a current, they are called electrolytes. Why do electrolytes conduct an electric current? The definitions of an acid, base, and salt have undergone several modifications in the history of chemistry. As knowledge about chemistry expanded, definitions of these words were also expanded to cover larger groups of compounds. The first part of this chapter traces the development of the definitions of these terms.
24: 1 ARRHENlUS THEORY
In 1887, a Swedish chemist, Svante Arrhenius, published a paper concerning acids and bases. He knew that solutions containing acids or bases conducted an electric current. Arrhenius tried to explain why. He concluded that these substances released charged particles when dissolved. He called these charged particles ions (wanderers). He concluded that acids were substances which separated (ionized) in water solution to produce hydrogen ions, H+, or free protons). He also believed that bases were substances which ionized to produce hydroxide ions, OH- in water solution.
HC1 ---> H+ + C1-
NaOH ---> Na+ + OH-
24::2 BRONSTED-LOWRY THEORY
As the knowledge of catalysts and nonaqueous solutions increased, it became necessary to redefine the terms acid base. In l923, an English scientist, T. M. Lowry, and a Danish scientist, J. N. Bronsted, independently proposed a new definition.
They stated that in a chemical reaction, any substance which donates a proton is an acid and any substance which accepts a proton is a base. When hydrogen chloride gas is dissolved in water, ions are formed.
HCl(aq) (acid) + H20(l) (base) ---> H3O+(aq) + Cl- (aq)
In this reaction, hydrogen chloride is an acid, and water is a base. Notice that the hydrogen ion has combined with a water molecule to form the polyatomic ion H30+, which is called the hydronium.
There is strong evidence that the hydrogen ion is never found free as H+. The bare proton is so strongly attracted by the electrons of surrounding water molecules that H30+ forms immediately. Consider the opposite reaction.
H30+(aq) + Cl-(aq) ---> HCl(aq) + H2O(1)
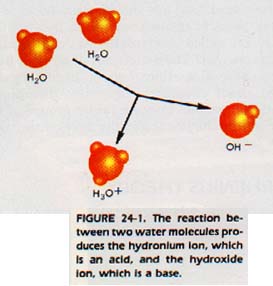
In this reaction, the H3O+ + ion is an acid. It acts as an acid because it donates a proton to the chloride ion, which is a base.
The hydronium ion is said to be the conjugate acid of the base, water.
The chloride ion is called the conjugate base of the acid, hydrochloric acid.
In general, any acid-base reaction is described as:
acid + base ---> conjugate base + conjugate acid
The conjugate base of an acid is the particle that remains after a proton has been released by the acid. The conjugate acid of a base is formed when the base acquires a proton from the acid.
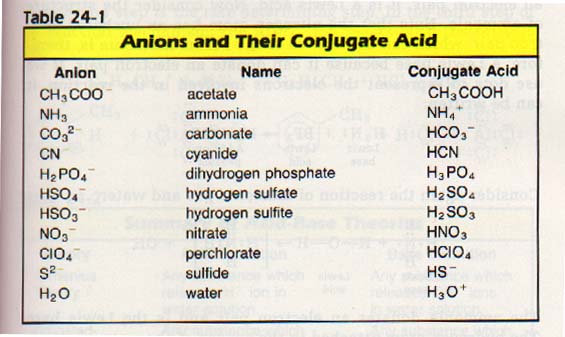
Table 24-1 contains a list of some anions and their conjugate acids.
Consider what happens when ammonia gas is added to water.
NH3(ag) + H20(1) ---> NH4+(aq) + OH-(aq)
base + acid ---> conjugate acid + conjugate base
In this reaction, water acts as an acid because it donates a proton to the ammonia molecule. The ammonium ion is the conjugate acid of ammonia, a base, which receives a proton from water. Hydroxide ion is the conjugate base.
24:3 LEWIS THEORY
In 1923, the same year that Bronsted and Lowry proposed their theories, another new idea appeared. Gilbert Newton Lewis, an American chemist, proposed an even broader definition of acids and bases. The same type of reasoning as Bronsted's and Lowry's led to his proposals.
However, Lewis focused on electron transfer instead of proton transfer. He defined an acid as an electron-pair acceptor, and a base as an electron-pair donor.
This definition is more general than Bronsted's. It applies to solutions and reactions which do not even involve hydrogen or hydrogen ions.

24:4 NAMlNG BlNARY AClDS
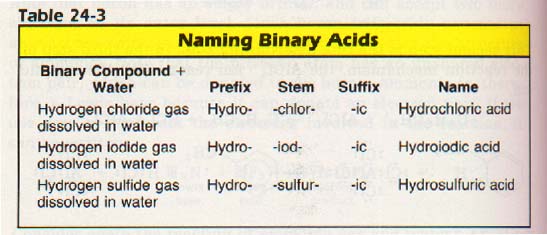
Binary acids are acids containing only two elements. If you look at Table 24-3, you will notice that the prefix is always hydro and the suffix is always -ic.
To name a binary acid, we determine what stem to use by finding what element is combined with hydrogen.
For instance, chlorine will have the stem -chlor-, and fluorine the stem -fluor-. To this stem, the prefix hydro- and the suffix -ic are added.
There are a few exceptions to the rule that binary acids begin with hydro- and end with -ic. One example is hydrocyanic acid, HCN, which really is ternary. These exceptions must be learned separately, but HCN is the only one we will mention.
24:5 NAMlNG TERNARY AClDS AND BASES
Ternary acids are acids which contain three elements. Most of the ternary acids we will be working with have oxygen as the third element. We find the stem by determining what element is combined with oxygen and hydrogen in the acid molecule.
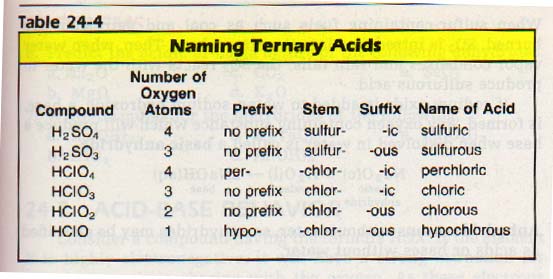
We determine the prefix (if there is one) and the suffix by the number of oxygen atoms in each molecule.
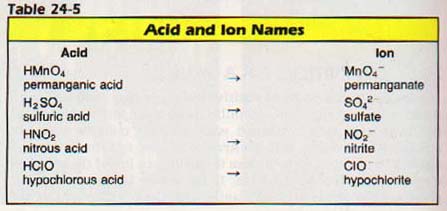
Generally, the most common form of the acid is given the suffix -ic. No prefix is used. Examples of common ternary acids are sulfuric (H2SO4), chloric (HC1O4), and nitric (HNO3).
If a second acid is formed containing the same three elements, but having less oxygen, this acid is given the suffix -ous. There is no prefix. Examples of these acids are sulfurous (H2SO3), chlorous (HC102), and nitrous (HNO2).
If a third acid containing still less oxygen is formed, it is given the prefix hypo- and the suffix -ous. An example is hypochlorous acid (HCIO).
Acids containing more oxygen than the common form are named by adding the prefix per- to the common name. For example, perchloric acid (HC104). See the examples in Table 24-4.
It is not possible, without previous knowledge, to know which form of an acid is most common. If the name of one form is known, the other ternary acids containing the same elements can be named. Bromine forms only two acids with hydrogen and oxygen: HBrO and HBr03. Instead of being named bromous and bromic acids, they are named hypobromous and bromic acids. The exception occurs because they contain the same number of oxygen atoms as hypochlorous and chloric acids.
The same pattern is followed in naming the ternary acids of the other halogens. Arrhenius bases are composed of metallic, or positively charged, ions and the negatively charged hydroxide ion.
Bases are named by adding the word hydroxide to the name of the positive ion. Examples are sodium hydroxide, NaOH, and calcium hydroxide, Ca(OH)2.
24:6 AClDlC AND BASIC ANHYDRIDES
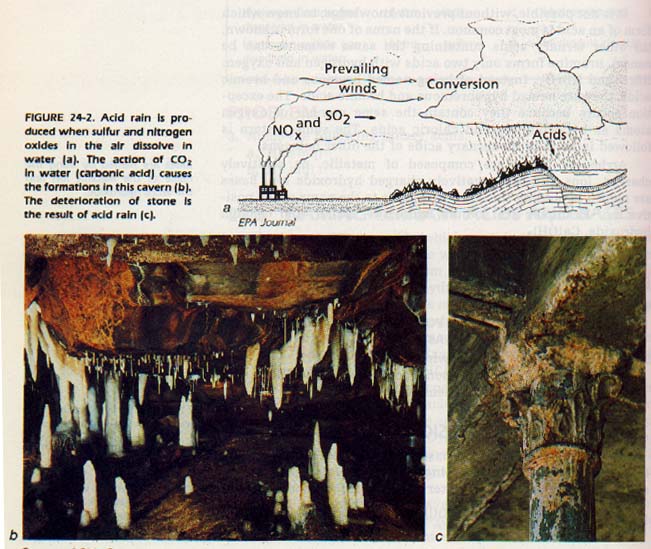
When sulfur dioxide is dissolved in water, sulfurous acid is formed. Any oxygen-containing substance which will produce an acid when dissolved in water is called an acidic anhydride.
SO2(g) (acid anhydride) + H2O(l) ---> H2SO3(aq) (acid)
When sulfur-containing fuels such as coal and petroleum are burned, SO2 is introduced into the atmosphere. Then, when water vapor condenses and rain falls, the SO2 reacts with the water to produce sulfurous acid.
If sodium oxide is added to water, sodium hydroxide, a base, is formed. Any oxygen containing substance which will produce a base when dissolved in water is called a basic anhydride.
Na2O(c) (basic anhydride) + H20(1) ---> 2NaOH(aq) base
Anhydride means without water, so anhydrides may be classified as acids or bases without water.
24:7 ACID-BASE BEHAVlOR
Consider a compound having the formula HOX. If the element X is highly electronegative, it will have a strong attraction for the electrons it is sharing with the oxygen. As these electrons are pulled toward X, the oxygen, in turn, will pull strongly on the electrons it is sharing with the hydrogen. The hydrogen ion, or proton, would then be lost easily. In this case, HOX is behaving as an acid.
If the element X has a low electronegativity, the oxygen will tend to pull the shared electrons away from X. The hydrogen will remain joined to the oxygen. Since in this case the formation of hydroxide ion, OH-, is likely, HOX is behaving as a base. We know that nonmetals have high electronegativities and metals low electronegativities.
We can conclude, then, that nonmetals will tend to form acids, and metals will tend to form bases.
Some substances can react as either an acid or a base. If one of these substances is in the presence of a proton donor, then it reacts as a base. In the presence of a proton acceptor, it acts as an acid. Such a substance is said to be amphoteric. Water is the most common amphoteric substance.
HCl (proton donor) + H2O (base) ---> H30+ + Cl-
NH3 (proton acceptor) + H2O (acid) ---> NH4+ + OH-
24:8 DEFlNlTlON OF A SALT
An acid is composed of positive hydrogen ions combined with negative nonmetallic ions.
Metallic bases are composed of negative hydroxide ions combined with positive metallic ions.
An acid reacts with a base to form a salt and water. The water is formed from the hydrogen ion of the acid and the hydroxide ion of the base. If the water is evaporated, the negative ions of the acid will unite with the positive ions of the base to form a new compound called a salt.
It would appear such a reaction should result in removal of all hydrogen and hydroxide ions from solution. The resulting solution should neither an acid nor a base. We could say that the solution is neutral (neither acidic nor basic).
The reaction of an acid and a base is called a neutralization reaction.
A salt is a crystalline compound composed of the negative ion of an acid and the positive ion of a base. For example, if equivalent amounts of chloric acid and sodium hydroxide react, sodium chlorate and water are formed.
HCl03(aq) + NaOH(aq) ---> NaClO3(aq) + H20(1)
acid + base ---> a salt + water
You may have already noted that there is a relationship between the name of an acid and the name of the salt it forms. Binary acids (prefix hydro-, suffix -ic) form salts ending in -ide. As an example, hydrochloric acid forms chloride salts. Ternary acids form salts in which -ic acids form -ate salts and -ous acids form -ite salts. Prefixes from the acid names remain in the salt names.

24:9 STRENGTHS OF AClDS AND BASES
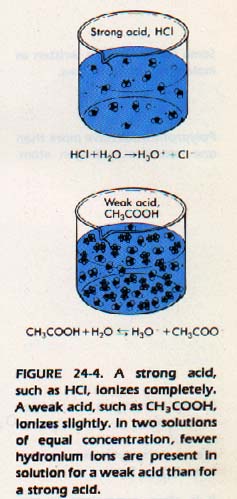
Not all acids and bases are completely ionized in water solution. An acid (such as hydrochloric) which is considered to ionize completely into positive and negative ions is called a strong acid. A base (such as sodium hydroxide) which is completely dissociated into positive and negative ions is called a strong base.
Some acids and bases ionize only slightly in solution. The most important base of this kind is ammonia. In water solution, this base ionizes only partially into NH4+ and OH-.
The major portion of the ammonia molecules remain unreacted. Such a base is called a weak base. Acetic acid ionizes only slightly in water solution. It is called a weak acid. A weak acid or a weak base is one which ionizes only slightly in solution.
24: 10 NET lONlC EQUATlONS
For reactions taking place in water, it is customary for chemists to write equations in the ionic form. In this method, only those ions taking part in the reaction are written.
Other ions present in the solution but not involved in the reaction are known as spectator ions and are not included in the equation.
In writing net ionic equations, dissolved salts are considered in their ionic form. We list other substances as molecules or atoms. The following rule must be observed when writing net ionic equations:
Substances occurring in a reaction in molecular form are written as molecules. Those occurring as ions are written as ions.
Weak acids and bases are written in molecular form while strong acids and bases should be written in the ionized form.
An acid may contain more than one ionizable hydrogen atom. Such an acid is called a polyprotic acid. Below are listed some "thumb rules" for deciding whether to use ions or molecules in writing net ionic equations. These rules are not applicable in all cases but work well in most reactions. However, if your equation must be exact, you should use a handbook. The handbook will help you determine whether substances are to be written as ions or as molecules.
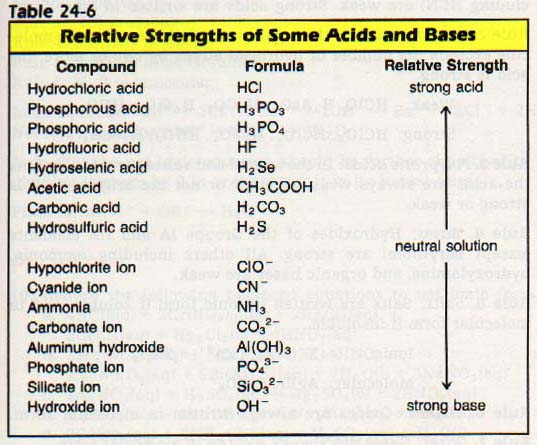
Rule 1. Binary acids: HC1, HBr, and HI are strong; all others (including HCN) are weak. Strong acids are written in ionic form.
Rule 2. Ternary acids: If the number of oxygen atoms in the molecule exceeds the number of hydrogen atoms by two or more, the acid is strong.
Weak: HCIO, H3As04, H2CO3, H4SiO4, HNO2
Strong: HCl03, HClO4, H2S04, HN03, H2Se04
Rule 3. Polyprotic acids: In the second and subsequent ionizations the acids are always weak, whether or not the original acid is strong or weak.
Rule 4. Bases: Hydroxides of the Groups IA and IIA elements (except beryllium) are strong. All others including ammonia, hydroxylamine, and organic bases are weak.
Rule 5. Salts: Salts are written in ionic form if soluble, and in molecular form if insoluble.
Ionic: K+ + C1-, Zn2+ + 2NO3-
Molecular: AgBr, BaSO4
Rule 6. Oxides: Oxides are always written in molecular form.
Rule 7. Gases: Gases are always written in molecular form.

24: 13 COMMON ION EFFECT
There are times when a chemist may wish to change the concentration of a specific ion in a solution. Such changes are often made by adding a new substance to the solution.
Acetic acid ionizes in a water solution to form acetate and hydronium ions. What will happen if we add some sodium acetate to the solution?
Sodium acetate dissociates into acetate, CH3COO-, and sodium ions.
NaC2H3O2 + Na+ + CH3COO-
Acetic acid ionizes into acetate and hydronium ions.
H20 + CH3COOH <---> H3O+ + CH3COO-
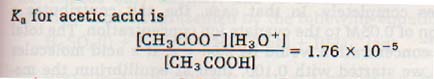
Ka is a constant, and does not change unless the temperature changes. If sodium acetate is added to an acetic acid solution, acetate ion concentration is increased and a shift in the equilibrium occurs.
Because there are more particles of CH3COO-, there will be more collisions between CH3COO- and H30+. Thus, the rate of reaction will increase toward acetic acid. Some of the excess acetate ions unite with hydronium ions to form molecular acetic acid and water. This reaction results in the removal of hydronium ions from solution (thus decreasing hydronium ion concentration).
The acetic acid concentration increases slightly. A new equilibrium is established with more acetate ions and fewer hydronium ions. Ka remains unchanged.
The acetate ion is common to both acetic acid and sodium acetate. The effect of the acetate ion on the acetic acid and solution is called the common ion effect. The addition of a common ion increases the concentration of one of the products of the ionization. Thus, the equilibrium shifts toward the opposite side in accordance with Le Chatelier's principle.
By adding acetate ions (sodium acetate), we have placed a stress on the system (a surplus of acetate ions). The system shifts so as to relieve that stress by reacting hydronium ions with the acetate ions. In the process acetate and hydronium ions are consumed and acetic acid molecules are produced.
SUMMARY
1. There are three common acid-base theories: the Arrhenius theory, the Bronsted-Lowry theory, and the Lewis theory.
2. Metallic oxides tend to form basic anhydrides, while nonmetallic oxides tend to form acidic anhydrides.
3. A Salt is a crystalline compound composed of the negative ion of an acid and the positive ion of a base.
4. A Strong acid ionizes completely in a water solution. A weak acid ionizes only slightly in a water solution.
5. In net ionic equations, only the reacting species are shown. Spectator ions do not appear.
8. The ionization of a weak acid or base is an equilibrium process.
7. Percent ionization is the amount ionized divided by the original amount multiplied by 100%.
8. A common ion represses the ionization of a weak electrolyte.
9. Polyprotic acids contain more than one ionizable hydrogen atom. Each successive ionization occurs to a lesser extent.
More on Acids, Bases, Salts:
For a PowerPoint presentation Click Here.
..... Batman Meets Deadly Acid
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page