Electrochemistry
![]()
Electrochemistry
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
ELECTROCHEMlSTRY
You press the button of your pocket flashlight and light (a form of radiant energy) is produced. You know already that the light results from the passage of an electric current through the flashlight bulb. Where does the electricity come from? The obvious answer is the battery. What then is a flashlight battery? How does it function?
If we plug the apparatus in Figure 27-1 into an electric outlet, the bulb does not light because the circuit is not complete. Whenever we complete the circuit with a conducting substance, the bulb does light. In the circuit shown, a conducting substance in the beaker would complete the circuit. We now have an easy way of observing which substances are conductors of electricity.
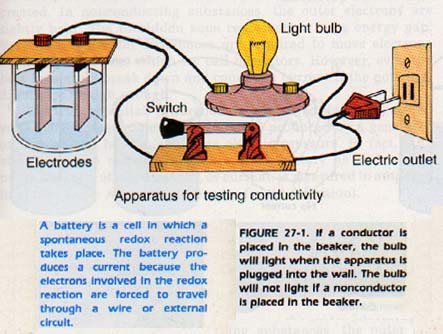
If we touch the two electrodes to a piece of copper or other metal, the bulb lights. Metals are conductors of electricity. Copper is one of the best conductors; for this reason it is used in electric equipment. If we touch the electrodes to a piece of glass or a sulfur crystal, the bulb does not light. Most nonmetallic solids, including salts, are nonconductors.
If we immerse the electrodes in water, benzene, alcohol, sugar solution or other solution of nonpolar substance, the bulb does not light. These liquids are nonconductors.
However, if we add a small amount of chloride (or any other salt), a small amount of hydrochloric or sodium hydroxide, the bulb lights. These solutions are conductors of electricity.
What materials conduct electricity in solution? Why do they conduct electricity?
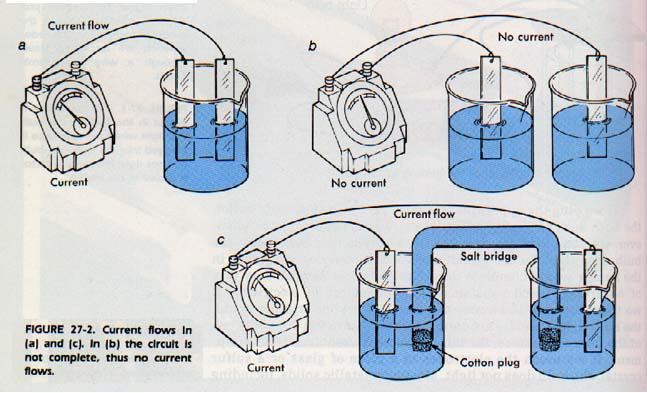
Let us take pieces of two dissimilar metals and connect to the terminals of a galvanometer. A galvanometer is an instrument for measuring electric current. The two pieces of meta are kept from making contact with each other and put into a salt solution. The galvanometer needle registers a flow of current. How is the current produced?
If we place the two metal plates into separate beakers containing salt solutions, as shown in Figure 27-2, no current flows. When we add the salt bridge to the system as shown, a current is produced. The salt bridge is a U-tube containing an ionic substance in solution. What is the function of the salt bridge? It connects the two separate solutions without mixing them. Can the solutions be mixed and still be used to: produce a current? In this chapter, we shall answer these and other related questions.
27:1 CONDUCTlVlTY AND POTENTlAL DlFFERENCE
Metals, in general, are excellent conductors of electricity. This statement is true whether the metal is in the solid state or the liquid state. Mercury, a liquid at room temperature, is used in scientific apparatus because of its excellent electric conductivity.
In Chapter 12, we found that the outer electrons of metal atoms are free to move if excited to the conduction band. If the potential energy of the electrons in the metal is raised, these electrons will flow to a point where their potential energy is lower. The flow or movement of electrons through a conductor is an electric current.
A difference in electric potential can be brought about in a number of ways. One way is to use a generator. Suppose we connect the two ends of a long wire to a generator. Electrons are added at one end of the wire and removed at the other end. When a potential energy difference is created at the ends of the wire, current flows.
Energy is required to create a potential difference and work is done when a current is made to flow through a wire.
Metals are excellent conductors of electricity because their electrons are free to move when a small potential difference is created. In nonconducting substances, the outer electrons are tightly held.
The forbidden zone represents a large energy gap. Very large potential differences are required to move electrons in these substances which we call insulators. However, even the best insulators break down and conduct a current if the potential difference is high enough.
Electric potential difference is measured in units called volts. The voltage (potential difference) produced by a generator or battery can be thought of as electric pressure. In fact, it is often easier to consider a generator or battery as an electron pump. The rate of electron flow, or current, is measured in amperes. An ampere is a coulomb/second.
27:2 ELECTROLYTIC CONDUCTlON
Solid acids, bases, and salts (for example, oxalic acid) are nonconductors. When any one of these substances is dissolved in water, the resulting solution is a conductor.
Those substances which are conductors in solution are known as electrolytes. Any substance which produces ions in solution is an electrolyte.
Salts are ionic even in the solid state; but a salt must dissolve in order for the ions to separate from each other and become free to move. (Conduction also occurs in a melted salt.) Acids and bases may be either ionic or molecular substances. However, when they dissolve in water, ions are formed.
Electrolytic conduction is possible because ions move freely in the liquid state.
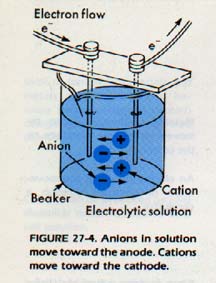
If we connect the conductivity device to a direct current, one of the electrodes will be negative and the other electrode will be positive. It is customary to call the negative electrode the cathode and the positive electrode the anode.
If we immerse the electrode in an ionic solution, positive ions in the solution will be attracted to the cathode. For this reason, positive ions are called cations, Negative ions will be attracted toward the anode. Consequently, they are called anions.
The movement of ions through a solution results in an electric current just as the movement of electrons in a metal results in a current.
27:3 ELECTRODE REACTlONS
What happens to a moving ion when it reaches the electrode to which it is attracted? We will consider molten sodium chloride, a system which contains only two kinds of ions and no other particles. We will use electrodes which are inert, that is, electrodes which do not react chemically with sodium or chloride ions.
The positive sodium ions, or cations, are attracted to the cathode. The cathode is made negative by the action of a generator which, in effect, pumps electrons into it. The electrons in the cathode are in a state of high potential energy.
The sodium ion has a positive charge. It has an attraction for electrons and an electron in a sodium atom would have a lower potential energy than an electron on the cathode. Thus, electrons move from the cathode (high potential energy) to the sodium ions (lower potential energy). At the cathode, the sodium ions will be converted into sodium atoms by the addition of an electron. This change is a chemical reaction and can be shown by an equation.
Na+ + e- ---> Na
Note that this chemical change represents a gain of electrons. We found in Chapter 26 that electron gain is called reduction. The chemical change which occurs at the cathode is always reduction. In this case, the sodium ion is reduced to sodium metal.
Now consider what happens at the anode. The anode has a positive charge and negative ions are attracted to it. The anode is positive because the generator is, in effect, pumping electrons out of it. Thus, electrons in the anode may be said to exist in a state of low potential energy.
Since the chloride ion has a negative charge, its outer electrons are in a state of higher potential. When chloride ions reach the anode, they give up electrons to the electron-deficient anode. Electrons move from a state of higher potential energy to a state of lower potential energy. We can show the chemical change which occurs at the anode by the following.
2C1- ---> Cl2 + 2e-
Note that in this reaction chloride ions lose electrons to become chlorine atoms which then combine to form Cl2 molecules. This reaction results in a loss of electrons. The name applied to a change in which electrons are lost is oxidation. The anode reaction is always oxidation.
We have shown the oxidation and reduction processes by separate equations because they take place at different points. However, these processes do not occur independently. The generator does not produce the electrons; it moves the electrons from one place to another. The electrons which the generator adds to the cathode are taken from the anode.
The reduction process cannot occur without the oxidation process going on at the same time. The role of the generator is to raise the potential energy of the electrons on the cathode.
These electrode reactions are called half-reactions. The anode reaction involves a loss of electrons and the cathode reaction involves a gain of electrons. Therefore, the electrons must balance when we add the two half-reactions. The overall reaction for the electrolysis of sodium chloride is
2Na+(l) + 2Cl-(l) ---> 2Na(l) + Cl2(g)
The process by which an electric current produces a chemical change is called electrolysis. Electrolysis of molten sodium chloride is the usual commercial process by which metallic sodium is produced. Chlorine gas is produced at the same time as a byproduct.
SUMMARY
1. An electric current is carried through a metal by the movement of electrons. An electric current is carried through a solution or molten salt by the movement of positive and negative ions.
2. When two points with a potential difference are connected by a conductor, an electric current will flow. An electric current is the flow of electricity through a conductor. It passes from the point of higher potential to the point of lower potential.
3. Current is measured with an ammeter or galvanometer, and is expressed in amperes
4. Voltage is the measure of potential difference. Voltage is measured with a voltmeter, and is expressed in volts (V).
5. Electrolyte solutions are electrically neutral, but contain ions which are free to move, as do molten ionic compounds. Cations are positive ions; anions are negative ions.
6. When ions migrating through an electrolytic cell reach an electrode, they undergo oxidation or reduction reactions.
7. A voltaic cell is a cell which produces an electric current and is composed of two dissimilar metals and an electrolyte.
8. Any oxidation-reduction reaction can (theoretically) be set up in such a way that current will be produced.
9. An oxidation-reduction reaction is a reversible reaction. Therefore, any change in temperature, pressure, or concentration will affect the flow of electric current.
10. The reduction potential of electrode reactions measures the relative strength of oxidizing and reducing agents.
More on Electrochemistry:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page