Nuclear Chemistry
![]()
Nuclear Chemistry
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
NUCLEAR CHEMlSTRY
In 1896, Henri Becquerel, a French physicist, found that matter containing uranium exposes photographic film. This fact led Becquerel's assistants, Pierre and Marie Curie, to an important discovery. They found that rays are given off by the elements uranium and radium.
Uranium and radium can be found in nature in an ore called pitchblende. This ore is mined in Canada, Colorado, and Germany. If some of this ore is placed near, but not touching a charged electroscope, the leaves become discharged. Substances which have the same effects as uranium and radium are called radioactive substances. The whole phenomenon is called radioactivity.
28: 1 NUCLEAR STRUCTURE
The rays produced by radioactive materials are a mixture of several particles and quanta of energy. The particles and quanta are given off by the nuclei of radioactive atoms during spontaneous nuclear decay. We say the decay is spontaneous because we have no control over it. The amount of energy released in a nuclear change is very large. It is so large that it cannot be a result of an ordinary chemical change.
Albert Einstein was the first to explain the origin of this energy (Chapter 1). From his theory, we see that mass and energy are equivalent. This statement can be expressed in the equation
E= mc2
E is the energy (in joules) released m is the mass (in kilograms) of matter involved c is a constant, the speed of light in meters per second.
The energy-mass change in normal chemical reactions is too small to be measured. However, we are familiar with the nuclear changes which involve a mass-energy interconversion. The large amount of energy released in splitting the nuclei of uranium or plutonium is measurable.
The nucleus of the uranium-238 atom contains 92 protons and 146 neutrons. These particles are bound, tightly in the nucleus. Acting alone, electrostatic attraction would allow the uncharged neutrons to float away.
Since protons all have the same positive charge, electrostatic forces should cause them to fly apart. Gravitational forces which keeps us at the surface of the earth, is not strong enough to hold the nucleus together. What force, then, holds the nucleus together?
Ideas explaining nuclear structure differ, but scientists agree on certain facts:
(1) The nuclear force which holds protons and neutrons together is effective for very short distances only. This distance is about the same as the diameter of the nucleus.
(2) Nucleons (protons and neutrons) have a property which corresponds to the spin of electrons.
(3) Electrons do not exist in the nucleus, yet they can be emitted from the nucleus.
28:2 SUBATOMlC PARTICLES
Nuclear scientists divide subatomic particles into two broad classes, leptons and hadrons. Current theory holds that leptons ("light" particles) are truly elementary particles. The electron is the best known lepton.
For every particle, a mirror-image particle called an antiparticle exists, or is believed to exist. Thus, there is an antielectron, called a positron, which is like an electron in every way except that it has a positive charge. Positrons are not common. They exist only until they collide with an electron. Such a collision is very likely in our world.
When the collision occurs, both particles are destroyed and two quanta of energy are produced. It is interesting to note that there is at least one particle which is its own antiparticle. This particle is the neutral pion. There are several other leptons.
In order to account for the emission of electrons from the nucleus, a neutral particle called a neutrino was postulated. The neutrino has been identified and found to be essentially massless. The muon and the tau, both much more massive than the electron, make up the rest of the lepton family. There is a neutrino for the muon and one predicted for the tau. There are antiparticles for all these particles.
The hadrons are subdivided into two groups, the mesons and the baryons. Protons and neutrons are baryons, as are a number of other short-lived particles. There are several kinds of mesons.
Mesons and baryons are made of smaller particles called quarks. There exists experimental evidence for four kinds of quarks: "up," "down," "charmed," and "strange." Two other kinds are believed to exist, "top" and "bottom." The names convey nothing about the properties of the quarks. They are just labels to identify different particles. Each quark comes in three "colors," red, blue, and green. Again, the "color" label refers only to a distinguishing property, not an appearance. Each kind of quark, of course, has an antimatter counterpart, an antiquark. Baryons are composed of three quarks, each of a different color. Mesons are composed of a quark and an antiquark of complementary color.
It is believed that quarks are held together by exchanging "particles" called gluons. The exchange of gluons between quarks is analogous to the exchange of photons by atoms. In a similar manner, the nucleons are held together in the nucleus by the exchange of pions. There are believed to be eight (or maybe nine) gluons, each of which is characterized by possessing one color and one anticolor.
The entire field of nuclear structure and the relationships of subatomic particles is a field of intense study. Much of the study is done using particle accelerators.
28:3 ACCELERATORS
Nuclear scientists use both linear and circular devices to accelerate particles. Both types operate using the charges on the particles. When the particle has achieved a very high speed (and thus a very high energy), it is aimed at a target material. The interaction which results as a consequence of the collision helps scientists understand the structure of the nucleus.
In a linear accelerator a particle is introduced using a device similar to the mass spectrometer ion-beam generator, Figure 28-3. The charged particle in motion will be affected by an electromagnetic wave. A radio wave is used to "push," or accelerate, the particle down the "pipe" forming the core of the accelerator.
The wave, however, is traveling at the speed of light. Since the particle is not traveling as fast, the wave tends to overtake the particle. If that were to happen, the particle would then decelerate. Therefore, there are uncharged tubes in the pipe called "drift tubes." The particle drifts through the tube until the next accelerating part of the radio wave is in the correct position.
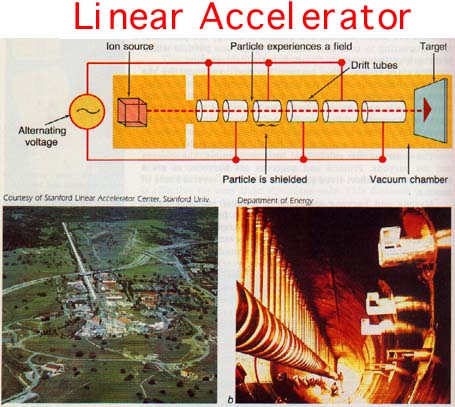
Careful design of the drift tubes as well as control of the radio wave frequency is important to assure maximum acceleration. The exit of the particle from the drift tube and the arrival of the accelerating part of the wave must coincide.
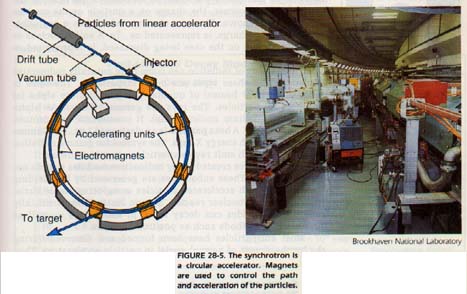
In a circular accelerator, the "pipe" through which the particles move is in the shape of a circle, or slightly modified circle, Figure 28-5. Particles are accelerated by electric fields in several locations around the ring. The path of the particles is confined to the ring by huge magnets surrounding the ring.
As the particles are accelerated, the magnetic field must be increased to keep the particles in the ring. Careful synchronization of acceleration and increasing magnetic fields is important. Hence, the device is called a "synchrotron." Recently, some experiments have involved two rings whose beams collide head-on. An accelerator is used to produce a stream of particles which is stored in one ring. The accelerator can then generate a second stream to collide with those already in the storage ring.
28:4 RADlOACTlVlTY
An electron, which has a negligible mass and a -1 charge, is represented as -1eo. A sulfur nucleus or atom, depending on the case being discussed, is represented as 16S32.
Three forms of radiation can come from naturally radioactive nuclei. Two of these types are made of particles. The third is made of rays (or beams) of quanta.
The particles are alpha, α, and beta particles, β. The rays are gamma rays, γ. An alpha particle, α, is a helium nucleus, 2He4, It consists of two protons and two neutrons. A beta particle, β, is an electron, -1e0. Gamma rays, γ, are very high energy electromagnetic rays (just above X-Rays). The symbol for gamma radiation is γ.
Nuclei which emit rays or particles are said to decay. Scientists have created many radioactive nuclides that do not exist in nature. These substances are generated by bombarding stable nuclei with accelerated particles or exposing stable nuclei to neutrons in a nuclear reactor, Section 28:10. These artificially radioactive nuclides can decay.
Most antiparticles have been formed and observed during the bombardment of normal nuclei in particle accelerators. They may also be formed in other ways. Other artificially radioactive nuclides decay by capturing one of the two 1s electrons outside the nucleus. The first energy level (n = 1) is sometimes called the K level. For this reason, this radioactive process is called K-capture.
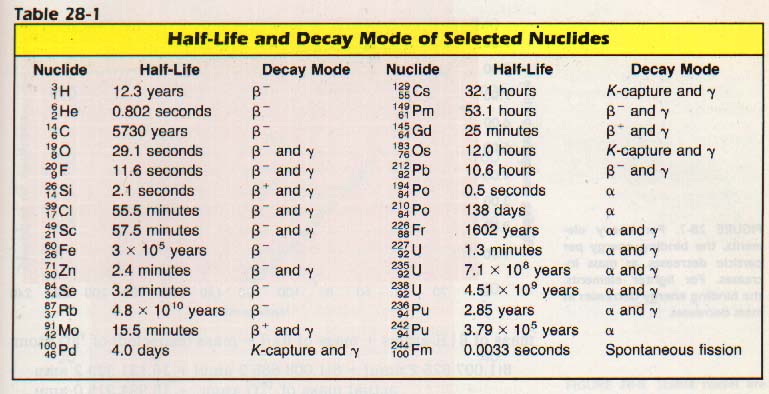
28:5 HALF-LIFE
The rate at which many radioactive nuclides decay has been determined experimentally. The number of atoms which disintegrate in a unit of time varies directly as the number of atoms present.
The length of time it takes for one-half of the atoms to disintegrate has been chosen as a standard for comparison purposes. This time interval is called the half-life.
28:7 TRANSMUTATIONS
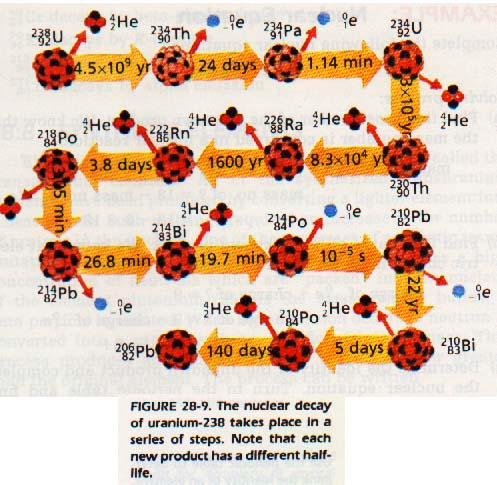
Nuclear reactions can result in the change of one element into another. If a reaction changes the number of protons in the nucleus, an atom with a different atomic number is obtained. This change is called transmutation. The most common isotope of natural uranium is 92U238. Uranium-238 decays by emitting an alpha particle, α, 2He4. The resulting nuclide contains two fewer protons and two fewer neutrons than 92U238. Thus, it has an atomic number of 90 and a mass number of 234.
28:10 FISSION
If a very heavy nucleus becomes too unstable, it breaks into two approximately equal parts. At the same time a large amount of energy is released. This process is called fission. A nucleus can be made unstable by bombardment with a number of particles, including neutrons.
The heaviest elements are the only ones which exhibit the phenomenon of fission. When a heavy nucleus breaks up, two heavy fragments plus one or more neutrons are emitted. One atom splits due to bombardment by a neutron. In the process of splitting, the atom gives off a neutron. In turn the neutron can cause a second atom to split.
This process continues until a very large number of the atoms present have reacted. Thus, the emitted neutrons produce a chain reaction. Since a large energy change is involved in the fission process, a chain reaction can serve as an energy source for various purposes.
A nuclear reactor is a device for controlling nuclear fission. Reactors can be designed to produce heat for electric power generation plants, or for propulsion units in ships and submarines. In nuclear reactor, the rate of the chain reaction is controlled very carefully. An uncontrolled nuclear chain reaction results in an explosion similar to a nuclear bomb, often incorrectly called an atomic bomb.
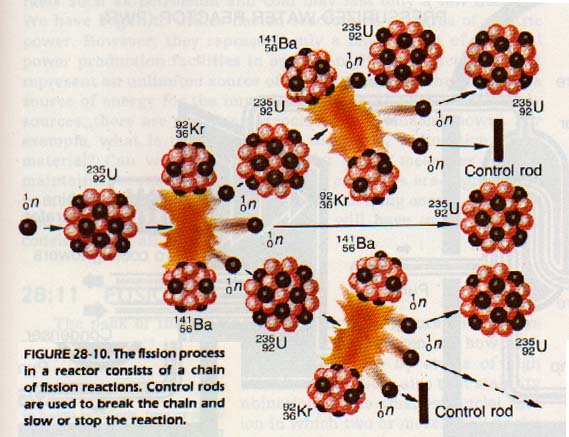
In a nuclear reactor, a fissionable element is used as fuel. Uranium and plutonium (itself a product of a reactor) can be used as fuels.
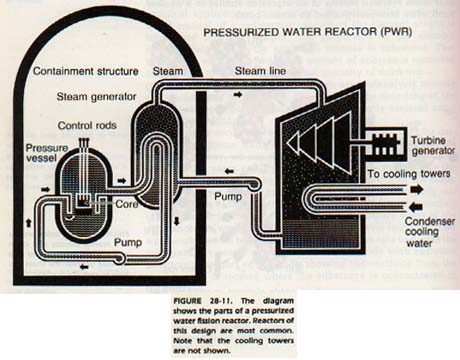

The demand for energy in the United States has outstripred our ability to produce new sources. Worldwide resources of fossil fuels such as petroleum and coal may last only a few decades. We have begun to utilize fission reactors as a source of electric power. However, they represent only a few percent of the total power production facilities in operation.
Fission reactors do not represent an unlimited source of energy. However, they do offer a source of energy for the immediate future. As with other energy sources, there are problems associated with nuclear power. For example, what is to be done with the highly radioactive waste material? Can we be sure that strict safety measures will be maintained at reactor sites?
European countries are aggressively pursuing nuclear power as a means of satisfying energy demands. The future of fission power reactors will have to be carefully considered by all people.
28: 11 FUSION
A nuclear reaction in which two or more small nuclei combine to form one larger nucleus is called a fusion reaction. We should therefore expect fusion reactions to produce much greater amounts of energy per particle than fission reactions. This prediction is supported by observation.
Scientists have harnessed the fission reaction in a controlled process on a small scale for limited power production. If we could harness the fusion reaction for power production, we would have a solution to energy problems for some time to come.
Some of the difficult problems associated with fission reactions as a power source would also be eliminated. The availability of "fuel" for nuclear fusion reactions is much greater than for fission reactions. Also, fusion reactions which are useful for power production do not result in radioactive waste products.
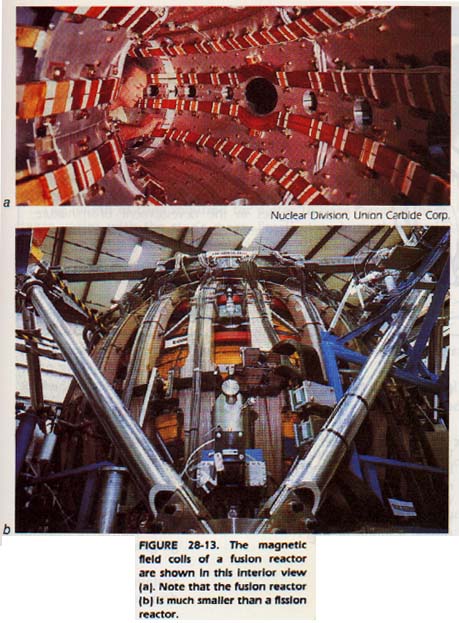
28:12 FUSION REACTORS
In order for two atomic nuclei to undergo fusion, they must come almost into contact. Recall that the nuclear force extends only about a distance equal to the diameter of the nucleus.
However, since all nuclei are positively charged, they tend to repel each other strongly. In order for fusion reactions to occur, the reactants must be held in the temperature range of 108 to 109oC for a suitable time. The energy requirements to achieve these temperatures are quite high. The length of time required depends upon how closely packed the particles are during that time. At these high temperatures, matter is in the form of plasma.
One major problem in designing a fusion reactor is containing the fuel at that temperature. Containers made from usual materials cannot be used. The plasma would lose heat to them so rapidly that fusion temperatures would never be reached.
Since plasmas are charged particles. they are affected by magnetic fields. By properly shaping a magnetic field, a plasma may be contained. As fusion occurs and the temperature rises, the pressure of a plasma causes it to expand.
With charged particles in the plasma moving around at tremendous velocities, the plasma itself generates electric currents. Both of these effects make leakage from the magnetic "bottle" a difficult problem to solve.
All of these problems are topics of present research. Who can predict what developments in this fascinating field will play in shaping our future!
SUMMARY
1. Radioactivity is the phenomenon of particle or quantum emission due to nuclear disintegration.
2. Radioactive decay is spontaneous; that is, it cannot be controlled.
3. Subatomic particles are divided into elementary particles called leptons and complex particles called hadrons.
4. Hadrons are believed to be made of particles called quarks.
5. Quarks and hadrons are thought to be held together by exchanging gluons and pions respectively.
g. Linear accelerators and synchrotrons are devices used by nuclear scientists to investigate nuclear structure by bombarding nuclei with high energy particles.
6. Naturally radioactive nuclides emit three kinds of radiation: alpha (helium nuclei), beta (electrons), and gamma (quanta of energy).
8. Many subatomic particles in addition to electrons and nucleons have been discovered. Some of these are classified as antimatter.
9. Each radioactive nuclide emits a characteristic radiation. Therefore, radioactive nuclides are extremely useful in the laboratory and in industry.
10. The half-life of a radioactive substance is the time it takes for one half of the atoms of a sample of the substance to disintegrate.
11. Three relationships can be used to predict the stability of nucIides:
(a) the binding energy per particle
(b) the neutron-proton ratio
(c) an even number of both protons and neutrons
12. The calculated mass defect of an atom indicates the transformation of mass into binding energy.
13. Binding energy is the energy needed to separate the nucleus into individual protons and neutrons.
14. The changing of one element into another is called transmutation.
15. In completing nuclear equations, both mass number and electric charge must be conserved.
l6. Fission is the splitting of a large, unstable nucleus into two smaller, approximately equal parts.
17. A nuclear reactor is a device for containing and controlling a fission reaction.
18. New elements may be synthesized by combining particles or nuclei with other nuclei.
19. Fusion is the combining of two or more small nuclei into one larger nucleus.
More on Nuclear Chemistry:
..... Obtain the Atomic Structure Notes
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page