Reaction Rate & Equilibrium
![]()
Reaction Rate & Equilibrium
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
REACTlON RATE AND CHEMlCAL EQUlLlBRlUM
In studying the properties of matter, chemists wish to find out both the physical and the chemical characteristics of a substance. They want to be able to describe the behavior of a given substance with many other substances. Does it react?
We have found that the change in free energy for a reaction can be used to answer that question. Of great importance, especially to the industrial chemist, is the answer to the question: How fast does it react?
Josiah Willard Gibbs discovered the relationship between entropy and enthalpy called the free energy. The amount of free energy after a spontaneous reaction is less than before the reaction.

Consider the reaction
2Ti0 ---> 2Ti + 02
ΔG = +495 kJ
Since the free energy change is positive, the reaction does not take place. Titanium(II) oxide does not spontaneously decompose at room temperature. It is said to be thermodynamically stable.
As we have seen, a negative free energy change, ΔG is negative, indicates a reaction will proceed spontaneously. However, some spontaneous reactions take place so slowly that it takes hundreds of years for any observable change to occur. For example, the ΔG for combustion of glucose, a common sugar, is
C6H12O6 + 6O2 ---> 6C02 + 6H20
ΔG = -2870 kJ
However, at room temperature, the reaction proceeds so slowly that sugar is said to be kinetically stable. Therefore, to predict whether a given spontaneous reaction will be useful, we must know the rate at which the reaction occurs as well as at what point equilibrium is established.
Consider what happens when we add an ice cube to flask of water. The temperature of the water drops. Heat is as the ice cube gets smaller and solid ice is converted into liquid water.
We may represent this process by an equation.
solid + heat ---> liquid
if we use a thermos bottle instead of a beaker and our cube is large enough, the temperature of the water will drop to OoC. After the temperature reaches OoC, we observe no melting of the ice. If we now add a piece of metal which has chilled to -20oC, we may be surprised to find that the ice grows larger Evidently the process can go either way. At OoC equilibrium exists.
solid + heat ---> liquid
The relative amounts of solid and liquid can be changed adding or removing a small amount of heat without changing temperature. We say that the water-ice mixture is in equilibrium.
23:1 REVERSlBLE REACTIONS
A chemist studies the structure of matter and the properties which result from this structure. Some of these properties are chemical reactions. Actually, the study of chemical reactions is the study of the breaking and forming of chemical bonds.
The formation of chemical bonds is a complex subject. So far we discussed only the simplest chemical reactions, those which to completion. A reaction goes to completion when all of one the reactants is used completely and the reaction stops.
Reactions of this kind go from reactants to products. Not all reactions go to completion. Consider the following reaction.
H2 + I2 ---> 2HI
The arrow means the reaction is read from left to right, but the equation is only partially correct. The bond between the hydrogen and iodine in the hydrogen iodide molecule is a weak bond. Therefore, hydrogen iodide breaks easily into hydrogen gas and iodine vapor. The following equation represents this reaction.
H2 + I2 <--- 2HI
Notice the direction in which the arrow points. This is read from right to left. We now combine the two equations.
H2 + I2 <---> 2HI (reversible reaction)
The first reaction is said to go from left to right. The second reaction is said to go from right to left. The combined equation represents a reversible reaction. Note that this use of the word reversible is different from the use in Chapter 18 concerning work.
23:2 REACTlON RATE
Suppose the product of a reversible reaction decomposes faster than the reactants form the product. Then there will always be more reactant than product. Here is an example. HI decomposes to H2 and I2 more rapidly than H2 unites with I2 to form HI.
There will always be more hydrogen and iodine than hydrogen iodide. Consider a flask containing hydrogen, iodine, and hydrogen iodide. The hydrogen iodide is decomposing rapidly, more rapidly than H2 and I2 can combine to produce it. The rate of disappearance of hydrogen iodide is defined to be the reaction rate.
H2 + I2 <--- 2HI (rate of disappearance of HI)
(Notice this reaction is the reverse reaction read from right to left.) The rate of appearance of hydrogen iodide is defined as the rate of the reaction from left to right.
H2 + I2 ---> 2HI (rate of appearance of HI)
Reaction rate is usually defined in terms of the rate of disappearance of one of the reactants. It can also be defined as the rate of appearance of one of the products.
The usual units for reaction rates are mol/dm3/s.
What we are actually measuring is the rate of change of concentration. If we know the two reaction rates, we can predict whether product or reactant will be in the higher concentration at equilibrium. We will now consider some factors which affect reaction rates.
23:3 NATURE OF REACTANTS
The nature of the reactants involved in a reaction will determine the kind of reaction that occurs. Reactions with bond rearrangement or electron transfer take longer than reactions without these changes.
Ionic reactions (such as double displacement and neutralization reactions) occur almost instantaneously. They are rapid because ions of one charge are attracted by those of opposite charge and ions collide frequently.
In a reaction between ions, no electron transfer is involved. Reactions between neutral molecules are slower than ionic reactions because electron transfer and bond rearrangement must occur.
As pointed out in Chapter 15, most molecular collisions are elastic. The molecules simply rebound and move away unchanged. However, some collisions do have enough energy to cause changes in the electron clouds of the colliding molecules.
When the change occurs, the colliding molecules may form an activated complex. The energy required to form the activated complex is known as the activation energy. If the activation energy is high, few collisions have enough energy to form the activated complex. As a result, the reaction may be so slow that it cannot be detected. Consider the following reaction.
CH3CH2Br + OH- ---> CH3CH2OH + Br-
The activated, complex is CH3CH2(OH)Br-.
From Figure 23-2 we can see that the activation energy is 89.5 kJ per mole or CH3CH2Br. We can also see that the enthalpy change for the reaction is -77.2 kJ.
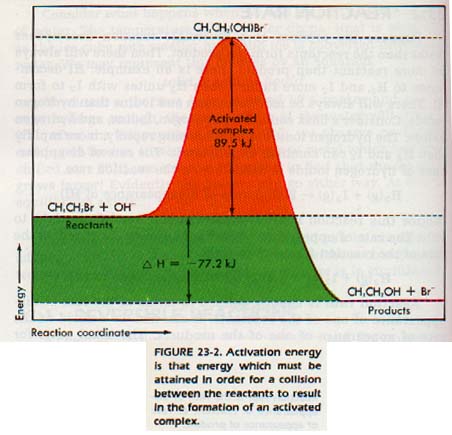
We can now see why some substances are kinetically stable even though many of their reactions may have negative free energy changes. These reactions have very high activation energies. Consequently, unless very strong reaction conditions are used, the reactions do not take place.
For example, hydrogen and oxygen can be kept in the same container at room temperature for ages without reacting to form water. Although the molecules collide, the activation energy will not be reached. If, however, the mixture is heated to 800oC, or a flame or spark is introduced, a violent reaction occurs. The heat, flame, or spark furnishes the activation energy. BAM!
Most reactions occur in a series of steps. Each step normally involves the collision of only two particles. Steps involving three ar more particles are unlikely. There is little chance of three or more particles colliding with the proper position and energy to cause a reaction.
If a reaction consists of several steps such as the following
A ---> B
B ---> C
C ---> final product
One of the steps will be slower than all the others. This step is called the rate determining step. The other faster steps will not affect the rate. The series of reaction steps that must occur for a reaction to go to completion is called the reaction mechanism.
23:4 CONCENTRATlON
Concentration refers to the quantity of matter that exists in a unit volume. For instance, in Chapter 5 we discussed the concentration in mol/liter of solution. We referred to this concentration as the molarity of the solution.
For a reaction to take place, the particles must collide. The Collision Theory.
If the number of particles per unit volume is increased, the chance of their colliding is also increased. Reconsider the equation
H2(g) + I2(g) ---> 2HI(g)
If we keep the concentration of the hydrogen molecules the same, we would expect doubling the concentration of iodine to double the number of collisions between iodine and hydrogen molecules. In turn, the reaction rate would double. Actual experiment confirms that the rate of reaction varies directly as the concentration of iodine. Written in equation form, it is
rate1 = k1[I2]
where the brackets around the I2 mean "mol/liter". What if the concentration of iodine remains constant, and the concentration of hydrogen is allowed to vary? We can say that the number of collisions and, therefore, the reaction rate, varies directly as the concentration of hydrogen molecules. We write
rate2 = k2[H2]
If We allow the concentration of both iodine and hydrogen to vary, what will happen?
If we double the concentration of hydrogen and also doubles the concentration of iodine, there will be four times as many molecules. What will the reaction rate be? There will be four times as many molecules per unit volume and four times as many collisions. The reaction rate is found to be quadrupled. We conclude, then, that the reaction rate varies directly as the product of the concentrations of hydrogen and iodine. We write
rate = k[H2] [I2]
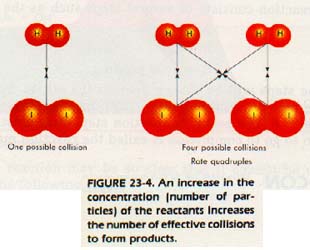
In this case, the constant k depends upon the size, speed, and kind of molecule involved in the reaction rate constant of the reaction.
It should be pointed out here that the actual mechanism of this reaction involves the breaking of the I-I bond before collision. However, it can be demonstrated mathematically that the same rate expression results.
An increase in the pressure on a gas results in a decrease in the volume occupied by the molecules. Since there are more molecules per unit volume, there has been an increase in concentration. Increasing the pressure on a gas, then, will also increase reaction rate.
Chemical reactions which take place at the interface between two phases are called heterogeneous reactions. An example of a heterogeneous reaction is zinc (a solid) dissolving in sulfuric acid (a liquid).
The reaction takes place on the surface of the zinc which is the interface between the two phases. If more surface is exposed, the reaction will take place more rapidly. Because of the unusual properties of surface molecules, their bonds are more easily broken. They react more readily than molecules within a solid. Increasing the surface area increases the number of surface molecules in the same space (increases concentration). Increasing surface area, then, increases the rate of a reaction.
23:5 TEMPERATURE
Reaction rate is determined by the frequency of collision between molecules and increases as the frequency of collision increases. According to the kinetic theory, the speed (kinetic energy) of molecules increases as the temperature increases.
Increased kinetic energy means that more collisions will occur and the reaction rate will increase.
However, the increase in reaction rate depends less on the increase in the number of collisions than it does on another factor. This other factor is the increase in the number of molecules which have reached activation energy.
Note from the graph in Figure 23-5 that molecules must collide with a kinetic energy sufficient to react. Otherwise a collision will not lead to reaction.
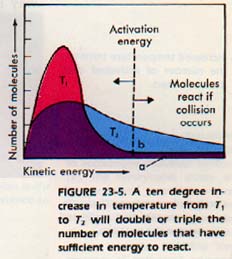
On the graph, the area under the curve indicates the number of molecules present. At temperature T1 few molecules have attained activation energy. At temperature T2, (T2 > T1) many more molecules have reached the activation energy.
The same number of molecules is present at this higher temperature. However, the fraction of molecules that have attained the activation energy is greater at the higher temperature T2.
Figure 23-6 is a graph which shows the energy changes involved in the reaction of hydrogen with oxygen. This graph can be thought of as a map of the potential energy possessed by the atoms and molecules taking part in a reaction.
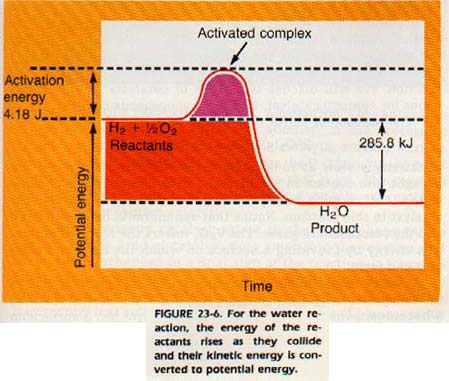
The gases, H1 and 02, are considered to have no potential energy (0 kJ). As two molecules approach, the kinetic energy of motion is transformed into the potential energy of repulsion of electron clouds. As more and more kinetic energy is transformed into potential energy, the line of the graph, which represents potential energy, rises.
If the molecules have enough kinetic energy to approach close enough to react chemically, they are said to be activated. Activated molecules form an activated complex.
From here, the reaction must go downhill in either direction. The activated complex may fall back on the left side and break into H2 and 02 molecules, or it may react (fall down on the right side).
The chance of falling either way is equal. If the activated complex does react, heat will be released and a molecule of water will be formed. The effect of raising the temperature is to produce more activated complexes through collision. Thus, with the increase in number of activated complexes, the number that will react will also increase.
An increase in temperature will increase the rate of any reaction. More activated complexes are formed because the number of collisions having the required activation energy increases.
23:6 CATALYSlS
A substance which increases a reaction rate without being permanently changed is called a catalyst.
Catalysis is the process of increasing rates of reaction by the presence of a catalyst. The catalyst remains (apparently) chemically unaffected throughout the reaction. It changes the reaction mechanism in such a way that the activation energy required is less than in the uncatalyzed reaction.
We will discuss two kinds of catalysts: the heterogeneous (or contact) catalyst, and the homogeneous catalyst. The reaction of sulfur dioxide gas with oxygen gas
2S02(g) + O2(g) ---> 2SO3(g)
is extremely slow at room temperature. If these two gases are brought into contact in the presence of solid vanadium(V) oxide, V205, the reaction is rapid. Vanadium(V) oxide is called the catalyst in this reaction.
Notice that vanadium(V) oxide is a solid and the reactants are gases. The V205 lowers the required activation energy by providing a surface on which the activated complex can form.
A reaction in which the reactants and catalysts are not in the same state is a heterogeneous reaction. The catalyst is called a heterogeneous catalyst. This kind of catalyst has a surface on which the substances can react. Platinum and other finely divided metals and metallic oxides are common examples of this kind of catalyst. Most heterogeneous catalysts work by adsorbing one of the reactants.
It might be correct to think of the contact catalyst as taking part in the reaction. Adsorption is the adherence of one substance to the surface of another. In the process of adsorbing a molecule, such as 02, the catalytic surface attracts the 02 molecule.
This attraction weakens the 0-0 bond to the point where the other reactant can break the 0-0 bond. The reaction then proceeds. Catalytic converters on automobile exhaust systems employ a contact catalyst.
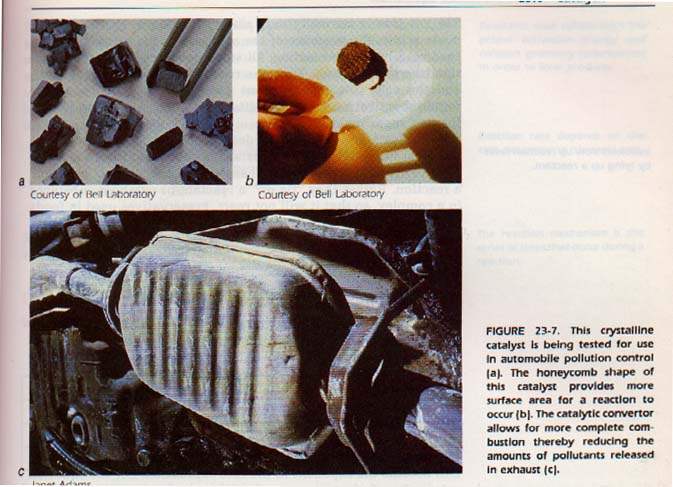
A homogeneous catalyst exists in the same phase as the reactants. This kind of catalyst does enter into the reaction, but is returned unchanged in a final step of the reaction. It forms an intermediate compound or compounds which react more readily than the uncatalyzed reactants. They react more readily because they require less activation energy. As an example of homogeneous catalysis, consider the hydrolysis of sucrose (cane sugar). The reaction is
C12H22011 (aq) (sucrose) + H2 0(1) ---> C6H1206(aq) (glucose) + C6H1206(aq) (fructose)
The reaction is normally very slow. If, however, the solution is made acidic, the presence of the acid causes the reaction to proceed readily. In the reaction, all substances are in aqueous solution (the same phase). Thus the reaction is a homogeneous one, and the acid is a homogeneous catalyst. The acid lowers the required activation energy by attacking the oxygen atom linking the two sugar molecules.
Catalysts are used a great deal in industry, as well as in the chemical laboratory. Other substances called inhibitors are also used to affect reaction rates. These substances do not "slow up" a reaction. Rather they "tie up" a reactant or catalytic substance in a complex, so that it will not react.
Preservatives used in foods and medical preparations are included to avoid spoilage. These substances are examples of inhibitors.
23:7 REACTlON MECHANlSM
At a given temperature, the rate of a reaction varies directly as the product of the concentrations of the reactants in the slowest step. For the reaction H2 + I2 ---> 2HI, the rate expression was rate = k[H2][I2].
For the general reaction A + B ---> C, the rate expression would be rate = k[A][B].
Hydrogen iodide decomposes into hydrogen and iodine. The equation is 2HI ---> H2 + I2.
How do we know if a reaction is a single-step? The only way to obtain accurate rate information is experimentally. As a result, the observation of reaction rates has given scientists an insight into the mechanisms of reactions.
Here is an example of a reaction and its mechanism:
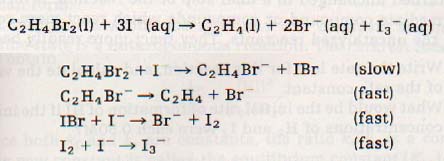
23:8 EQUlLlBRlUM CONSTANT
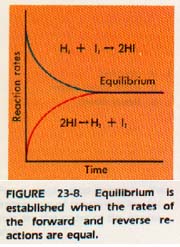
We have seen that the reaction of H, and I, to form HI is an equilibrium reaction. As the reaction proceeds, the reaction rate of the hydrogen and iodine reaction decreases. As H2 and I2 are used, fewer collisions between H2 and I2 molecules occur per unit time.
The reverse reaction, the collision of two HI molecules to form H2 and 12, does not occur initially because the concentration of HI is zero.
However, as the concentration of HI increases, the reverse reaction, the decomposition of hydrogen iodide, steadily increases. Equilibrium is attained when the rates of the two opposing reactions are equal. The rates of the forward and reverse reactions of the hydrogen iodide reaction are written
rate of forward reaction = kf[I2][H2]
rate of reverse reaction = kr[HI]2
Here is the Grand Formula for The Big K of Equilibrium:

23:9 LE CHATELIER'S PRlNClPLE
The conditions affecting equilibrium are temperature, pressure, and concentration of either reactants or products.
If a system is in equilibrium and a condition is changed, then the equilibrium will shift toward restoring the original conditions.
As you learned in Chapter 17, this statement is called Le Chatelier's Principle. Le Chatelier first described the effect of stress (change of conditions) upon systems at equilibrium. His principle holds for reaction equilibrium as well.
If stress is put on a reversible reaction at equilibrium, the equilibrium will shift in such a way to relieve the stress.
Let us see how it applies.
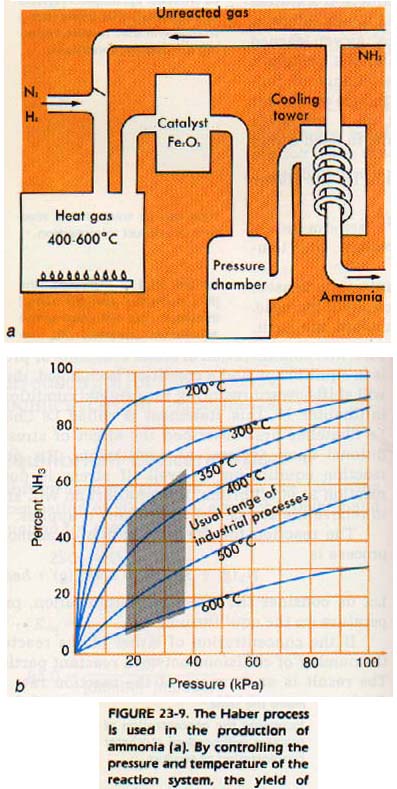
The reaction for the preparation of ammonia by the Haber process is N2(g) + 3H2(g) ---> 2NH3(g) + heat
Let us consider the effect of concentration, pressure, and temperature on the equilibrium.
If the concentration of either of the reactants is increased, the number of collisions between reactant particles will increase. The result is an increase of the reaction rate toward the right. As the amount of NH3 increases, the rate of the reverse reaction will also increase. However, the net result to the system as a whole is to shift the equilibrium toward the right. That is, more product is produced.
If the pressure is increased, the same effect is noted. That is, more product is formed. Consider the situation if the pressure is doubled. The concentrations of nitrogen, hydrogen, and ammonia are all doubled.
The equilibrium expression for this reaction shows the concentration of ammonia is squared.
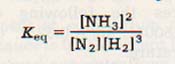
The reverse reaction must then speed up by a factor of 4. On other hand, the concentration of hydrogen is cubed. Further, it is multiplied by the concentration of nitrogen. Doubling the pressure then should increase the rate of the forward reaction by 23 x 2, or 16 times! The net result is clearly an increase in product.
In the reaction H2 + Cl2 <---> 2 HCl, again all substances are gases.
Pressure would not shift the equilibrium, as the rate in each direction would be affected the same way. Pressure, of course, has an effect only on the gases in a reaction. A reaction taking place in solution would be unaffected by pressure.
Both the forward and reverse reactions at equilibrium are speeded by an increase in temperature. However, their rates are increased by different amounts. Also, the value of the equilibrium constant itself is changed by a change in temperature. One easy way to predict the shift in an equilibrium subjected to a temperature change is to consider heat as a reactant or product.
In the Haber Process:
N2 + 3H2 <---> 2NH3 + heat
heat is produced when hydrogen and nitrogen react. If heat is considered to be a product, the addition of heat (a product) will increase the concentration of the product. The equilibrium will be shifted to the left. In the Haber process, the reverse action (the decomposition of ammonia) is favored by the addition of heat.
SUMMARY
1. A reversible reaction is a reaction in which products may reform reactants.
2. Reaction rate is the rate of disappearance of one of the reactants of a reaction or the rate of appearance of one of the products.
3. Four factors influence reaction rate: nature of reactants, concentration, temperature, and catalysis.
4. More reactive materials require less activation energy. Therefore, they react more rapidly.
5. Most reactions take place in a series of steps called the reaction mechanism.
6. Concentration is the quantity of matter present in a unit volume. The symbol [ ] means concentration in units of moles per cubic decimeter (liter).
7. Increasing the concentration of a reactant increases the rate of reaction by increasing the number of collisions. Increasing the pressure of a gas or the surface area of a heterogeneous reactant has the same effect on the reaction rate.
8. Increasing the temperature increases the rate of reaction. There are more frequent collisions and more of the collisions involve sufficient energy to form the activated complex.
9. A catalyst is a substance which causes an increase in reaction rate without being permanently changed.
10. A reaction in which the reactants and catalyst are not in the same phase is a heterogeneous reaction. The catalyst used is called a heterogeneous catalyst. A homogeneous catalyst is one which is in the same phase as the reactants.
11. Analysis of rate data can give chemists an insight into reaction mechanisms.
12. At a given temperature, the rate of a single-step reaction varies directly as the product of the concentrations of the reactants.
13. The equilibrium constant, K(eq) is the ratio of the forward rate constant, kf, divided by the reverse rate constant, kr
14. Le Chatelier's principle states that if an equilibrium system is subjected to stress, then the equilibrium will shift to relieve the stress.
15. Equilibrium constants for gas phase reactions can be expressed in terms of the partial pressures of the reactants and products.
More on Equilibrium:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page