Solubility Product and the pH Scale
![]()
Solubility Product and the pH Scale
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
SOLUTIONS OF ELECTROLYTES
Thus far in our investigation of electrolytes we have dealt mainly with acids and bases. Solutions of salts also involve equilibria. It is possible, through a reaction called hydrolysis, for an apparently neutral salt to dissolve in water and produce an acidic or basic solution. In this chapter we will investigate the interaction between electrolytes and water.
25:1 SOLUBILITY PRODUCT CONSTANT
Silver bromide is an ionic compound that is only slightly soluble in water. When an ionic compound is in a solution so concentrated that the solid is in equilibrium with its ions, the solution is saturated. The equilibrium equation for a saturated solution of silver bromide is
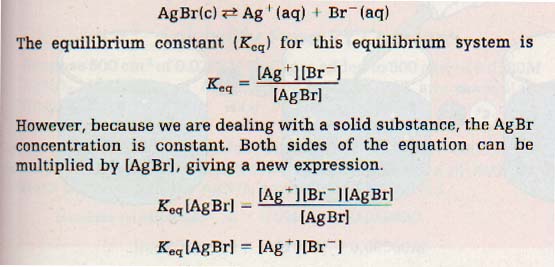
and

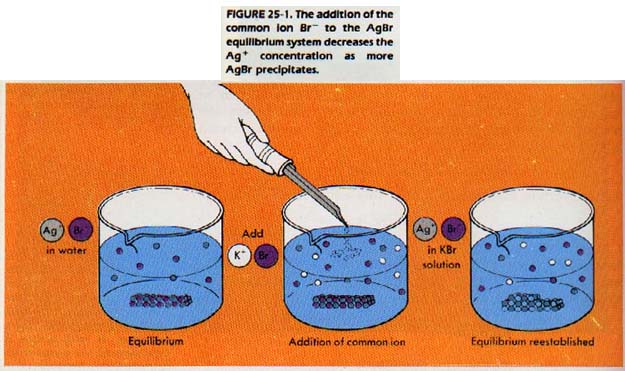
The increased number of bromide ions collide more frequently with silver ions. The equilibrium is thus shifted toward the solid silver bromide. When equilibrium is again established, the concentration of silver ion, [Ag+], has decreased. At the same time, the concentration of bromide ion, [Br-], has increased.
Solid silver bromide has precipitated out, and the Ksp 7.70 x 10-13, ha been reestablished.
This reaction is an example of the common ion effect. The addition of a common ion removes silver ion from solution, and causes the equilibrium to shift toward the solid silver bromide. We could also say the addition of a common ion decreases the solubility of a substance in solution.
25:2 IONIZATION OF WATER
Conductivity experiments have shown that water ionizes according to the equation
H2O(l) + H2O(l) <---> H3O+(aq) + OH-(aq)
Even though it does conduct a current, pure water is a poor conductor. An electric current is carried in a solution by the ions in the solution. Since pure water is a poor conductor of electricity, it must contain few ions. Thus, pure water must ionize only slightly.
In pure water, the concentration of H30+ is equal to the concentration of OH-. Each molecule of H20 that ionizes produces one ion each of H30+ and OH-.
Since water ionizes, it should be possible to find an equilibrium constant for this reaction.

25:3 pH SCALE
The ionization of water is so slight that it is almost never considered in the actual production or use of acids and bases. Why, then, was it introduced? Knowledge of the ion product constant for water has enabled chemists to develop a simple acidity scale, called the pH scale. The scale can be used to indicate the basicity as well as the acidity of any water solution. The pH scale is a measure of hydronium ion concentration.
The concentration of H30+ is expressed as powers of 10, from 10-14 to 10o. This method is a convenient way to indicate the [H30+].
We could simplify this expression even further by writing only the exponent. For instance, 10-7 would be written as -7. However, the negative sign is undesirable. If [H30+] = 10-7, the log of [H30+] = -7.
If, however, we take the negative log of [H30+], we get the desired +7. The negative log of [H30+] is the pH.
Note that as the hydronium ion concentration increases and a neutral solution is made more acidic, the pH goes from 7 toward O. If the pH of a solution falls between 7 and 14, the solution is basic.
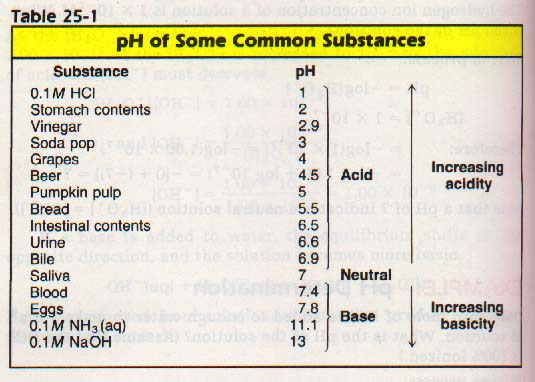
Table 25-1 indicates the pH of several common solutions. Note for those solutions which exist in the body, the maintenance of the pH shown is vital to life.
Suppose we wished to know the pH of a solution which was basic or about which we knew only the hydroxide ion concentration. We can use the ion product constant of water to find the relationship between pH and hydroxide ion concentration.
25:4 HYDROLYSIS
A salt is composed of positive ions from a base and negative ions from an acid. When a salt dissolves in water, it releases ions having an equal number of positive and negative charges. Thus, a solution of a salt should be neither acidic nor basic.
Some salts do form neutral solutions, but others react with water (hydrolyze) to form acidic or basic solutions.
There are four kinds of salt solutions we will discuss.
If potassium chloride is dissolved in water, a neutral solution results. Each ion from the salt, K+ and C1-, is hydrated with no apparent reaction (except hydration). Water ionizes very slightly to form H3O+ and OH- ions.
The solution will, therefore, contain ions from the salt and ions from the water. In solution, the positive potassium ion could unite with the negative chloride ion or the negative hydroxide ion. However, potassium chloride is a salt which ionizes completely in water.
Potassium hydroxide is a strong base which also ionizes completely. The same reasoning applies to C1- and H30+. No reaction occurs. The four ions remain in solution as ions, and the solution is neutral.
The ions produced by the salt of a strong acid and strong base do not react with water, and the [H3O+] remains equal to the [OH-].
Such a solution has a pH of 7. No hydrolysis occurs.
If we test a solution of aluminum chloride, a salt, we will find that the solution is acidic, not neutral as expected. With the exception of the metals of Groups IA and IIA, metallic hydroxides are all weak bases and in fact have low solubilities.
The positive ions of such metals are strongly hydrated and give up a proton readily from the associated water molecule. When AICl3 is dissolved in water, the aluminum ions become hydrated.
A1Cl3 + 6H20 ---> AI(H20)63+ + 3C1-
Remember that the action of a salt with water to form an acidic or basic solution is called hydrolysis.
25:5 BUFFERS
Many of the fluids in your body must be maintained within a very narrow pH range if you are to remain healthy. There are also many instances in laboratory and industrial chemistry when the maintenance of a certain pH is important.
In both cases, the end is accomplished in the same way: the creation of a buffer system.
A buffer system is a solution which can absorb moderate amounts of acid or base without a significant change in its pH.
25:6 INDICATORS
In the laboratory, how do chemists know whether they are using an acidic, basic, or neutral solution? They could taste the solution to determine whether it was bitter or sour. However, this method is inexact as well as dangerous because most solutions are poisonous or corrosive.
They could experiment to determine what reactions the solution undergoes, but the experiment might take too much time. The chemist generally uses a pH meter to determine the degree of acidity in a solution. This electronic device indicates the pH of a solution directly when its electrodes are immersed in the solution. We will investigate the operation of the pH meter in Chapter 27.
There are times when a pH meter may not be available, or its use may not be convenient. Therefore, sometimes substances called indicators are employed. Indicators are weak organic bases and acids whose colors differ from the colors of their conjugate acids or bases. When indicators are added to a test solution, the color that results is related to the pH of the solution. A number of indicators and their color changes are listed in Table A-ll of the Appendix.
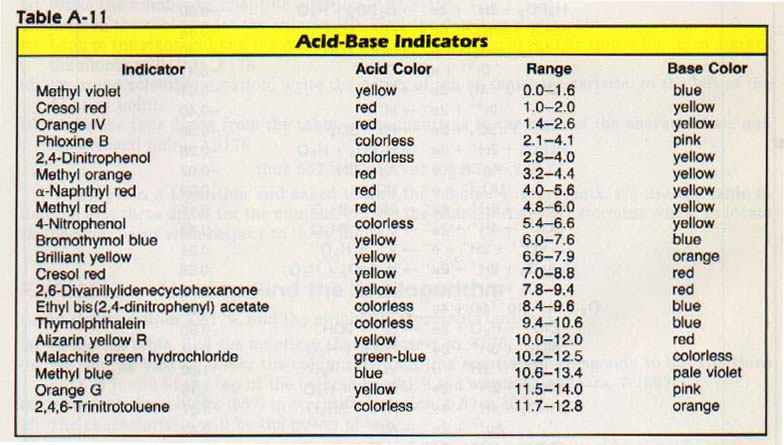
There are, however, limitations to the use of indicators. Solutions in which they are to be used successfully must be colorless. Otherwise, the color of the solution may mask the color changes of the indicator. Another important limitation is the ability of the human eye to distinguish a slight color change. For any given indicator, we can notice a color change over only a very narrow range of the pH scale.
To test for pH over a wide of the pH scale, many indicators must be used. However, definite pH, any change in [H30+] or [OH-] can be detected by use of one properly chosen indicator.

25:7 TITRATION
Analytical chemists often need to find the concentration a solution. Titration is an analytical method in which a solution is used to determine the concentration of another ion. A standard solution is one for which the concentration known.
If an acid is added to a base, a neutralization reaction occurs. The acid unites with the base to form a salt and water.
acid + base ---> a salt + water
For example, acetic acid can be neutralized by sodium hydroxide to form sodium acetate and water. By adding an indicator, we can see at exactly what point complete neutralization occurs.
To carry out a titration, a buret is filled with a standard solution. A small amount of indicator is added to a measured amount of the solution of unknown concentration to be titrated. The buret is opened and the standard solution is allowed to flow (with stirring) into the solution to be titrated.
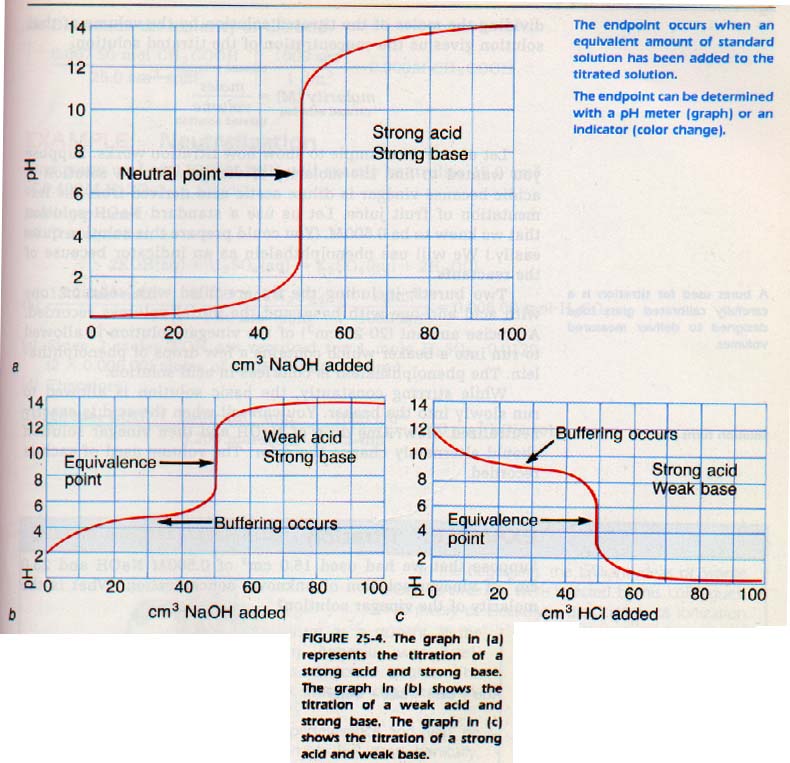
Eventually a color change occurs. The color change indicates the neutralization point. At that point (the endpoint) an equivalent amount of standard solution has been added to the solution titrated.
The endpoint may also be detected using a pH meter. If the pH meter is connected to a chart recorder, a graph of pH versus ml of titrating solution added is obtained. The endpoint corresponds to the middle of that portion of the graph showing a very large change in pH with the addition af a small amount of titrating solution.
The graph shown in Figure 25-4 is for the titration af a strong acid by a strong base. Weak electrolytes show a slightly different shape, shifted toward the pH of the stronger reactant.
The moles of standard solution can be calculated by multiplying the volume of standard solution used by its molarity.
moles (std sol) = volume (std sol) X molarity (std sol)
The moles in the titrated solution of unknown concentration are then found using the coefficients in the balanced equation. Then, dividing the moles of the titrated solution by the volume of solution gives us the concentration of the titrated solution.
molarity (M) = moles/volume
SUMMARY
1. The solubility product constant, Ksp and the ion product constant for
water, Kw are special cases of the equilibrium constant.
2. The pH of a solution is equal to -log [H30+].
3. Hydrolysis is the reaction of a salt with water to form an acidic or basic solution.
4. Buffer solutions are capable of absorbing moderate amounts of acid and/or base without a significant change in their pH value.
5. Indicators are weak acids and bases used to indicate the pH of a solution.
6. Acids and bases react with each other to form a salt and water. This type of reaction is called a neutralization reaction.
7. Titration is a laboratory process used to find the concentration of a substance in solution.
More on Electrolytes:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page