Oxidation-Reduction
![]()
Oxidation-Reduction
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
OXIDATION-REDUCTlON
In one method of classifying chemical reactions there are basically two different types. In the first type, ions or molecules react with no apparent change in the electronic structure of the particles. In the second type, ions or atoms undergo changes of electronic structure. Electrons may be transferred from one particle to another.
On the other hand, the sharing of the electrons may be somewhat changed. The second type of reaction involving electron changes is called an oxidation-reduction reaction. It is these "redox" reactions which we will now discuss. Before we indicate what oxidation-reduction reactions are, we will briefly indicate what they are not.
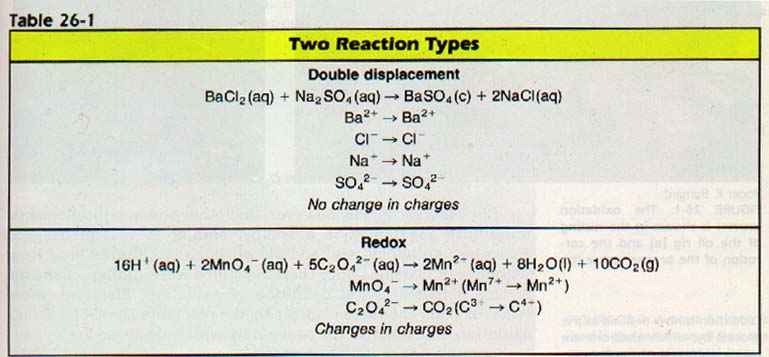
In the BaSO4 reaction in Table 26-1, the substances are all ionic. Since there is no change in the charge of these ions the reaction, there are no electron changes. This reaction is not an oxidation-reduction reaction.
The production of a (BaS04) is nearly always a result of a non-redox reaction. Most acid-base reactions are also the non-redox type. Since nearly every other kind of reaction is an oxidation-reduction reaction, redox reactions are important in the laboratory. They are also important in life processes and in industry.
26:1 OXIDATION
The term oxidation was first applied to the combining of oxygen with other elements. There were many known instances of this behavior. Iron rusts and carbon burns. In rusting, oxygen combines slowly with iron to form Fe2O3. In burning, oxygen unites rapidly with carbon to form CO2. Observation of these reactions gave rise to the terms "slow" and "rapid" oxidation.
Chemists recognize, however, that other nonmetallic elements unite with substances in a manner similar to that of oxygen. Hydrogen, antimony, and sodium all burn in chlorine, and iron will burn in fluorine.
Since these reactions were similar, chemists formed a more general definition of oxidation. Electrons were removed from each free element by the reactants O2 or Cl2.
Thus oxidation is defined as the process by which electrons are apparently removed from an atom or ion.
26:2 REDUCTION
A reduction reaction was originally limited to the type of reaction in which ores were "reduced" from their oxides. Iron oxide was "reduced" to iron by carbon monoxide.
Copper(II) oxide could be "reduced" to copper by hydrogen. In these reactions, oxygen is removed, and the free element is produced. The free element can be produced in other ways.
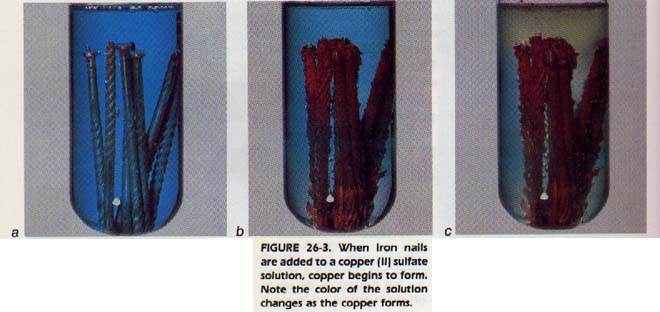
An iron nail dropped into a copper(II) sulfate solution causes a reaction which produces free copper. An electric current passing through molten sodium chloride produces free sodium. The similarity between oxidation and reduction reactions led chemists to formulate a more generalized definition of reduction.
By definition, reduction is the process by which electrons are apparently added to atoms or ions.
LEO GER
Lose Electrons Oxidation, Gain Electrons Reduction

26::3 OXlDlZlNG AND REDUClNG AGENTS
In an oxidation-reduction reaction, electrons are transferred. All the electrons exchanged in an oxidation-reduction reaction must be accounted for. It seems reasonable, therefore, that both oxidation and reduction must occur at the same time in a reaction. Electrons are lost and gained at the same time and the number lost must equal the number gained.
The substance in the reaction which gives up electrons is called the reducing agent. The reducing agent contains the atoms which are oxidized (the atoms which lose electrons). Zinc is a good example of a reducing agent. It is oxidized to the zinc ion, Zn2+
The substance in the reaction which gains electrons is called the oxidizing agent. It contains the atoms which are reduced (the atoms which gain electrons). Dichromate ion, Cr2072-, is a good example of an oxidizing agent. It is reduced to the chromium ion, Cr3+.
If a substance gives up electrons readily, it is said to be a strong reducing agent. Its oxidized form, however, is normally a poor oxidizing agent. If a substance gains electrons readily, it is said to be a strong oxidizing agent. Its reduced form is a weak reducing agent.
26:4 OXlDATlON NUMBERS
How is it possible to determine whether an oxidation-reduction reaction has taken place? We do so by determining whether any electron shifts have taken place during the reaction. To indicate electron changes, we look at the oxidation numbers of the atoms in the reaction.
The oxidation number is the charge an atom appears to have when we assign a certain number of electrons to given atoms or ions. Any change of oxidation numbers in the course of a reaction indicates an oxidation-reduction reaction has taken place.
For example, suppose iron, as a reactant in a reaction, has an oxidation number of 2+. If iron appears as a product with an oxidation number other than 2+, say 3+, or O, then a redox reaction has taken place.
26:5 ASSIGNING OXlDATlON NUMBERS
We have already seen in Chapters 4 and 10 how to predict possible oxidation numbers. Oxidation numbers are assigned according to the apparent charge of the element.
To determine the apparent charge, you may find it helpful to consult the electron dot structure for the substance. However, the electron dot structure you draw will not give you the complete answer; it only helps you to visualize an atom, ion, or molecule.
26:6 RULES FOR ASSIGNING OXlDATION NUMBERS
The following general rules have been made to enable you to determine the oxidation numbers more easily.
Rule 1. The oxidation number of any free element is O. This statement is true for all atomic and molecular structures: monatomic, diatomic, or polyatomic.
Rule 2. The oxidation number of a monatomic ion (Na+, Ca2+, A13+, C4+ is equal to the charge on the ion. Some atoms have several different possible oxidation numbers. For example, iron can be either 2+ or 3+; tin, 2+ or 4+.
Rule 3. The oxidation number of each hydrogen atom in most compounds is 1+. There are some exceptions. In compounds such as lithium hydride (LiH), hydrogen, being the more electronegative atom, has an oxidation number of 1-.
Rule 4. The oxidation number of each oxygen atom in most compounds is 2-.
Rule 5. The sum of the oxidation numbers af all the atoms in a particle must equal the apparent charge of that particle.
Rule 6. In compounds, the elements of Group IA and Group IIA and aluminum have positive oxidation numbers numerically equal to their group number in the periodic table.
SUMMARY
1. An oxidation-reduction reaction involves an apparent transfer of electrons from one particle to another.
2. Oxidation is the process by which electrons are apparently removed from an atom or group of atoms.
3. Reduction is the process by which electrons are apparently added to atoms or groups of atoms.
3. Any substance in a reaction which loses electrons is a reducing agent.
4. Any substance in a reaction which gains electrons is an oxidizing agent.
5. If a substance gives up electrons readily, it is a strong reducing agent. Its oxidized form is usually a poor oxidizing agent.
6. If a substance acquires electrons readily, it is a strong oxidizing agent. Its reduced form is usually a poor reducing agent.
7. Oxidation number is the charge an atom appears to have when we assign a certain number of electrons to that atom.
8. Six rules for assigning oxidation numbers:
a. The oxidation number of any free element is O.
b. The oxidation number of any single-atom ion is equal to the that ion.
c. The oxidation number of hydrogen is usually 1+.
d. The oxidation number of oxygen in most compounds is 2-.
e. The sum of the oxidation numbers of all the atoms in a particle equal the apparent charge of that particle.
f. In compounds, elements of Group IA and Group IIA and all have an oxidation number numerically equal to their group in the periodic table.
9. In all chemical reactions, charge, number and kind of atoms, and number of electrons are conserved. Knowing these quantities, you can do a redox equation.
10. Redox reactions are more easily balanced by splitting the equation into half-reactions.
More on Redox:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page