Periodic Table
![]()
Periodic Table
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
PERIODIC TABLE:
The element sodium reacts violently with water. Potassium reacts still more violently with water. An experienced chemist can predict that the elements rubidium, cesium, and francium will react in a similar manner. How can this prediction be made? Such predictions can be made because all of these elements have similar outer energy level structures. They have been placed in the first column of the periodic table because they have a similar structure.
EARLY ATTEMPTS AT CLASSIFICATION OF THE ELEMENTS:
Early in the 19th century, scientists began to seek ways to classify the elements. One attempt at classification was by Johann Dobereiner, a German chemist, in 1817. Dobereiner found that the properties of the metals calcium, barium, and strontium were very similar. He also noted that the atomic mass of strontium was about midway between those of calcium and barium. He formed what he termed a triad of these three elements. Later, Dobereiner found several other groups of three elements with similar properties.
In l863, John Newlands, an English chemist, suggested another classification. He arranged the elements in order of: increasing atomic masses. He noted that there appeared to be repetition of similar properties every eighth element. Therefore he arranged the elements known at that time into seven groups seven each. Newlands referred to his arrangement as the of octaves.
10:2 MENDELEEV'S PERlODlC TABLE
Just six years after Newlands' proposal, Dmitri Mendeleev, a Russian chemist, proposed a similar idea. He suggested, as had Newlands, that the properties of the elements were a function of their atomic masses.
However, Mendeleev felt that similar properties occurred after periods (horizontal rows) of varying length. Although he placed seven elements each in his first two periods, he placed seventeen elements in the next two.
In 1871, Mendeleev and the German chemist Lothar Meyer, working alone, made an eight-column table of the elements. Mendeleev had to leave some blank spots in order to group all the elements with similar properties in the same column.
To explain these blank spots, Mendeleev suggested there must be other elements that had not yet been discovered. On the basis of this arrangement, Mendeleev predicted the properties and atomic masses of several elements which were unknown at the time. These elements have now been discovered, and Mendeleev's have been found to be very nearly correct.
In Mendeleev's table, the elements were arranged in order their increasing atomic masses. The table showed that the properties of the elements are repeated in an orderly way. Mendeleev regarded the properties of the elements as a periodic function of their atomic masses. This statement was called the periodic law.
10:3 MODERN PERlODlC LAW
There was a problem with Mendeleev's table. If the elements were arranged according to increasing atomic masses, tellurium and iodine seemed to be in the wrong columns. Their properties were different from those of other elements in the same column. However, they were next to each other. Switching their positions put them in the columns where they belonged according to their properties.
If the switch were made, Mendeleev's basic assumption that the properties of the elements were a periodic function of their atomic masses would be wrong. Mendeleev assumed that the atomic masses of these two elements had been poorly measured. He thought that new mass measurements would prove his hypothesis to be correct. However, new measurements simply confirmed the original masses.
Soon, new elements were discovered, and two other pairs showed the same kind of reversal. Cobalt and nickel were known by Mendeleev, but their atomic masses had not been accurately measured. When such a determination was made, it was found that their positions in the table were also reversed.
When argon was discovered, the masses of argon and potassium were reversed. Henry Moseley found the reason for these apparent exceptions to the rule. As a result of Moseley's work, the periodic law was revised.
It now has as its basis the atomic numbers of the elements instead of the atomic masses. Today's statement of the periodic law is the properties of the elements are a periodic function of their atomic numbers.
10:4 MODERN PERlODlC TABLE
The atomic number of an element indicates the number of protons in the nucleus of each atom of the element. The atomic number also indicates the number of electrons surrounding the nucleus.
Certain electron arrangements are periodically repeated. We can place elements with similar electron configurations in the same column. We can also list the elements in the column in order of their increasing principal quantum numbers. This table is called the periodic table of the elements.
We construct our periodic table in this manner. We will use the diagonal rule (Section 9:13) to determine the order of filling the sublevels. Each s sublevel contains two electrons. Each p sublevel contains six electrons arranged in three pairs, or orbitals. Each d sublevel contains ten electrons in five orbitals. Each f sublevel contains fourteen electrons in seven orbitals. We then align the elements with similar electron configurations.
The first configuration, hydrogen (Z = 1), consists of one electron in the Is sublevel. The second configuration, helium (z = 2), consists of two electrons in the Is sublevel.
These two electrons completely fill the 1s sublevel. The third element, lithium (Z = 3), has two electrons in the Is sublevel and one electron in the 2s sublevel. Lithium is similar to hydrogen in that it has only one electron in its outermost sublevel.
Therefore, we will place it in the same column as hydrogen. The next element, is beryllium (Z = 4), has two electrons in the Is sublevel and two electrons in the 2s sublevel. It might seem to belong in the column with helium. However, the two electrons in helium's outermost level fill that level.
The two electrons in the 2s sublevel of beryllium do not fill the second level. Recall that the n = 2 level has a p sublevel, as well as its s sublevel.
Even though the beryllium and helium configurations are similar, beryllium starts a new column next to lithium.
Boron (Z = 5) has a configuration composed of two 1s electrons, two 2s electrons, and one 2p electron. It heads a new column. Carbon (Z = 6), nitrogen (Z = 7), oxygen (Z = 8), and fluorine (a= 9) atoms come next.
They have structures containing two, three, four, and five electrons, respectively, in the 2p sublevel. Each of these elements heads a new column.
The atoms of neon (Z = 10), the tenth element, contain six 2p electrons. The second column = 2) is now full, so neon is placed in the same column as helium.
Sodium atoms (Z = 11) have the same outer electron configurations as lithium atoms, one s electron (3sl). Thus, sodium is placed under lithium. The elements magnesium (Z = 12) through argon (Z = 18) have the same outer structures as the elements beryllium through neon.
They are also placed in the appropriate columns. Atoms of potassium (Z = 19) and calcium (Z = 20) have outer structures that are similar to the atoms of sodium and magnesium.
10:5 TRANSlTlON ELEMENTS
The scandium (Z = 21) configuration introduces a new factor into the arrangement. It has two electrons in the outer level (4s2) and is similar to the calcium configuration. However, the scandium atom has, in addition to a filled 4s sublevel, one electron in the 3d sublevel.
It is, therefore, placed in a new column, which is labeled (IIIB). For the atoms of elements titanium (Z = 22) through nickel (Z = 28), additional electrons are added in the 3d sublevel.
For all these elements, however, the outer level is the 4th level so they are placed in the fourth row. Each of these elements heads a new column (column IIIB to VIIIB).
Note that the atoms of copper and zinc have filled inner levels. All structures in column IB have filled inner levels and one electron in the outer level. All structures in column IIB have filled inner levels and two electrons in the outer level. In columns IIIA through VIIIA, electrons are added to the p sublevel until there are a total of eight electrons in the outer level.
The next electron is added to the next s sublevel whether the inner level is filled or not. The process is continued until all of the elements are placed in the main part of the table.
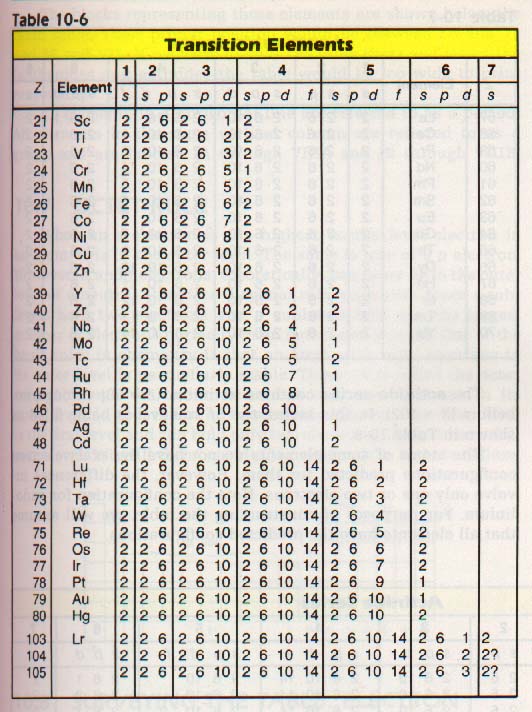
10:6 THE LANTHANlDES AND ACTlNlDES
The lanthanide series contains the elements lanthanum (Z = 57) through ytterbium (Z = 70). All of these elements have a predicted structure with two electrons in the outer level. In this series, electrons are being added to the 4f sublevel instead of to a sublevel of the sixth or outer level.
The actinide series contains actinium (Z = 89) through nobelium (Z = 102). In this series, the 5f sublevel is being filled.
The atoms of some elements do not have the exact electron configurations predicted for them. However, the differences involve only one or two electrons. Note the configuration for gadolinium. For purposes of constructing the table, we will assume that all elements have the predicted configurations.
The actinides in their proper position would be between 56 and 71 and 88 and 103. If we were to split the table there and insert the and actinides the table would be too wide to print .
All elements in a horizontal line are referred to as a period. Elements in the same vertical column are referred to as a group and are labeled IA through VIIIA and IB through VIIIB.
16:7 OCTET RULE
When an s electron is the highest energy level electron in an atom, it is in the outer level. The same is true of a p electron. However, d and f electrons, theoretically, can never be in the outer level of a neutral atom (see diagonal rule, page 167).
Since s Sublevels hold two electrons and p sublevels hold six, the largest number of electrons normally in an outer level is eight.
One of the basic rules in chemistry is that an atom with eight electrons in its outer level is particularly stable. This rule is called the octet rule.
Although the helium atom has only two electrons in its outer level, it, too, is one of these stable elements. Its outer level is the first level and can hold only two electrons. Thus, it has a full outer level. We will then consider the octet rule to include helium.
10:8 SURVEYING THE TABLE: ELECTRON CONFIGURATIONS
The periodic table was originally constructed by placing elements with similar properties in a column. We now know that an atom's chemical properties are determined by its electron configuration. By reversing the procedure in which the table was constructed, the table may be used to "read" the configuration of an element.
Thus, the written configuration of any element in Group IA will end in sl. This configuration means that the outer level of each atom of Group IA elements contains one electron. The coefficient of s1 is easily found from the table because the number of the period indicates the energy level. For example, potassium is in the fourth period of Group IA. Thus, the written electron configuration for the outer level of potassium is 4s1. The superscript, in s1, indicates the group number. The coefficient, in 4s1, indicates the period number.
For Groups IIIB through IIB, the endings are d4 through d10 preceded by a coefficient which is one less than the period number. For the lanthanides, the endings are fl through f14 preceded by a coefficient which is two less than the period number.
For transition elements, remember that the d sublevel is always preceded by an s sublevel that is one quantum number higher.
To understand some of the exceptions to the diagonal rule, it is necessary to know that there is a special stability associated with certain electron configurations in an atom. You already know that an atom with eight electrons in the outer level has this stability. An atom having a filled or half-filled sublevel is also stable.
Thus, chromium is predicted to have two electrons in its 4s sublevel and four electrons in its 3d sublevel. Actually, it has one electron in its 4s sublevel and five electrons in its 3d sublevel. Note that one electron is shifted between two very closely spaced sublevels.
The atom thus has two half-full sublevels instead of one full sublevel and one with no special arrangement. Copper has a similar change. Copper is predicted to have two 4s electrons and nine 3d electrons. Actually, it has one electron in its 4s sublevel and ten electrons in its 3d sublevel.
One full and one half-full sublevel make an atom more stable than one full sublevel and one with no special arrangement as predicted. Most of the exceptions from predicted configurations can be explained in this way. Sheesh!
10:9 METALS AND NONMETALS
Groups IA and IIA of the periodic table contain the most active metals. Many of the columns in the table have family names. Group IA, except hydrogen, is called the alkali metal family. Group IIA is called the alkaline earth metal family.
On the other side of the table are the nonmetals, in Groups VI, VIIA, and VIIIA. Group VIA is called the chalcogen family. Group VIIA is known as the halogen family. The elements of Group VIIIA are called the noble gases.
We are all familiar with typical metallic properties. Metals are hard and shiny. They conduct heat and electricity well. Nonmetals are generally gases or brittle solids. Their surfaces are dull and they are insulators. Chemists use the electron structure of elements to classify them as metals or nonmetals.

One characteristic of metals is that they have only a few electrons in the outer level. Nonmetals have more electrons in the outer level. There are exceptions.
However, as a general rule, elements with three or less electrons in the outer level are considered to be metals. Elements with five or more electrons in the outer level are considered to be nonmetals.
There are some elements which have properties of both metals and nonmetals. These elements are called the metalloids. Silicon, an element used in the manufacture of microcomputer chips, is a metalloid. On the periodic table, you will note a heavy, stairstep line on the right side. This line is a rough dividing line between metals and nonmetals. As you might expect, the elements along the line are generally metalloids.
The elements of Groups IB through VIIIB are called the transition elements. Since all atoms of transition elements have one or two electrons in the outer level, they all show metallic properties. The elements 57 through 70, and 89 through 102 have a similar characteristic.
These atoms have two electrons in the outer level as predicted and are therefore classified as metals. The elements of Groups IIIA through VA include both metals and nonmetals. At the top of the table, each of these groups contains nonmetallic elements. The metallic character of the elements increases toward the bottom of the table, and the last member of each family is distinctly metallic.
10:10 REVIEW: PERlODlC TABLE
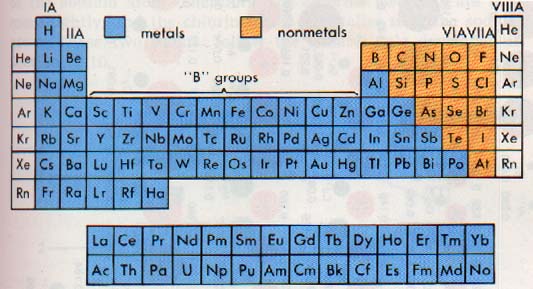
We can now look at the periodic table as a whole. Metals are located on the left and nonmetals on the right. Note again that most of the elements are metallic that is, their atoms contain one, two, or three electrons in the outer energy level. The most stable atoms are those of the noble gases.
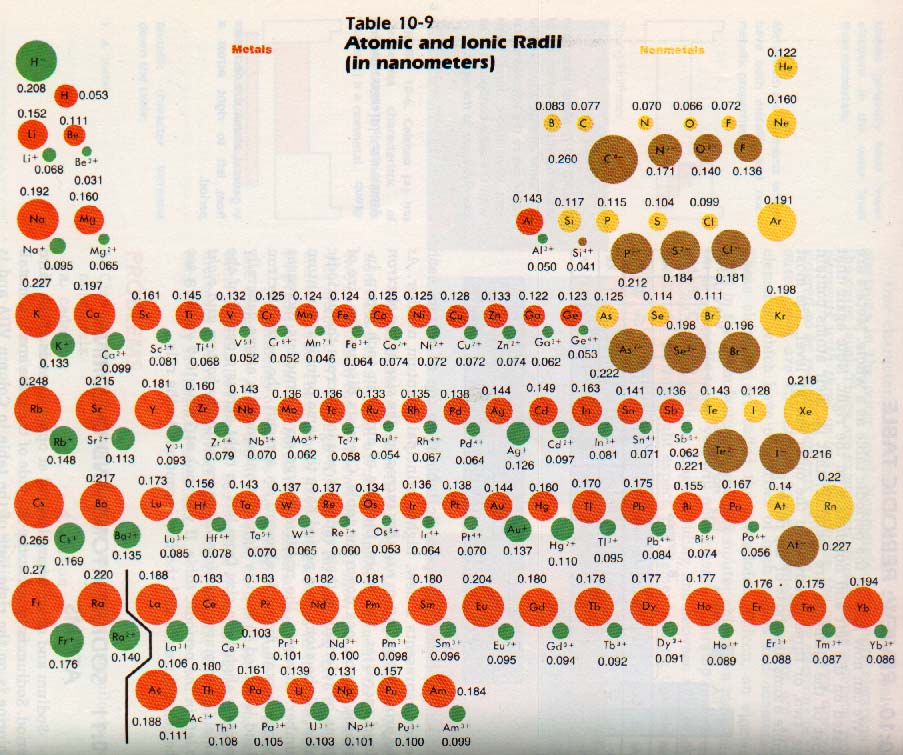
As you look at the periodic table from top to bottom each period represents a new, higher principal quantum number. As the principal quantum number increases, the size of the electron cloud increases. Therefore, the size of atoms in each group increases as you look down the table.
Chemists discuss the size of atoms by referring to their radii. As you look across the periodic table, all the atoms in a period have the same principal quantum number. However, the positive charge on the nucleus increases by one proton for each element. As a result, the outer electron cloud is pulled in a little tighter. Consequently, atoms generally decrease slightly in size from left to right across a period of the table. In summary, atomic radii increase top to bottom and right to left in the periodic table. If you look at Table 10-9 you can see the general trends and the few exceptions to the rule.
10: 11 SODlUM ATOMS AND CHLORlNE ATOMS
Sodium and chlorine are located at opposite ends of the third period. Sodium is found at the left side of the table and is a metal. Chlorine is on the right side of the table in Column VIIA and is a nonmetal.
Both sodium and chlorine have partially filled third levels. The outer electrons which take part in reactions are separated from the positively charged nucleus by two inner energy levels. These two inner levels are filled (ten electrons).
The chlorine nucleus contains seventeen protons; the sodium nucleus contains only eleven protons. The outer electrons of the chlorine atom are attracted by six more protons than are the outer electrons of the sodium atom. Therefore, the chlorine electrons are held more tightly, and the chlorine atom is smaller than the sodium atom. These two atoms, follow the general rule developed in Section 10:10.
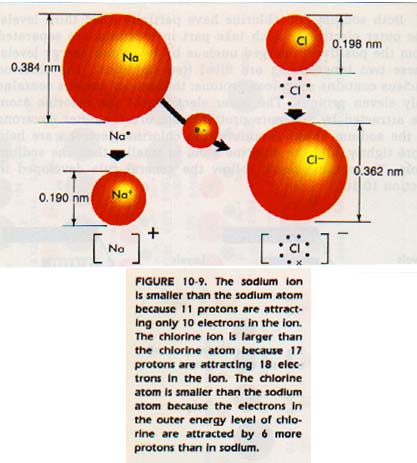
10: 12 SODIUM IONS AND CHLORlDE lONS
In general, when atoms unite to form molecules or compounds, their structures become more stable. Sodium and chlorine form the compound sodium chloride, or common table salt.
The sodium atom holds its single outer 3s electron loosely. When chlorine and sodium react, the more positive chlorine nucleus removes the outer electron from the sodium atom. (The Rip-off). The sodium atom has lost an electron.
The sodium ion has eleven protons, but only ten electrons, so it has a 1+ charge. Since the positively charged nucleus is now attracting fewer electrons, the sodium ion is smaller than the sodium atom.
The sodium ion is stable because its new outer level, 2s22p6 is the same as the outer level of the stable noble gas, neon.
The chlorine atom has gained an electron. The seventeen protons in its nucleus are now attracting eighteen electrons. The chloride ion thus has a 1- charge. Also, the chloride ion is larger than the chlorine atom. The ion is stable because it has the same outer level configuration as the noble gas, argon.
The compound formed from sodium ions and chloride ions does not consist of sodium chloride molecules. Instead, each cube of sodium chloride is a collection of equal numbers of Na+ and C1- ions.
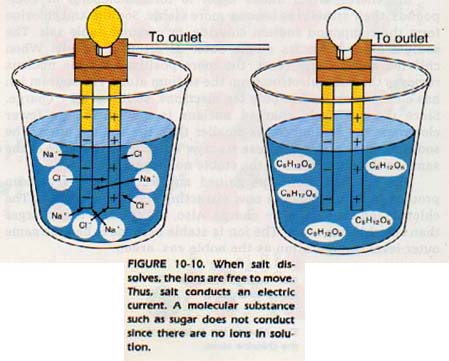
When salt is dissolved or melted, it will carry an electric current since the ions are free to move. However, a solid salt crystal will not carry an electric current because the individual ions are tightly bound. The mobility of electric charge completes a circuit. Thus, the substance is able to conduct a current.
We have noted that the sodium ion is smaller than the sodium atom. The magnesium ion is even smaller than its atom. In losing two outer electrons, the unbalanced nuclear positive charge is larger than the negative charge on the electron cloud. The cloud shrinks in size.
The nonmetals, sulfur and chlorine, form ions which are larger than their respective atoms. These elements gain electrons to form ions. The elements silicon and phosphorus do not gain or lose electrons readily. They tend to form compounds by sharing their outer electrons.
We can now look at some trends which will apply to any row of the periodic table. In general, metallic ions, on the left and in the center of the table, are formed by the loss of electrons. They are smaller than the atoms from which they are formed.
Nonmetallic ions are located on the right side of the table, they are formed by the gain of electrons and are larger than the atoms from which they are formed. The metallic ions have a stable outer level which resembles the noble gas at the end of the preceding period.
Nonmetallic ions have an outer level resembling the noble gas to the right in the same period.
10: 13 PREDlCTlNG OXlDATlON NUMBERS
Those electrons which are involved in the reaction of atoms with each other are the outer and highest energy electrons. You now know about electron configurations and the stability of atoms with noble gas structures. Thus, it is possible for you to predict what oxidation numbers atoms will have.
Consider the metals in Group IA. Each atom has one electron in its outer level. The loss of this one electron will give these metals the same configuration as a noble gas.
Group IA metals have an oxidation number of 1+. Note that the hydrogen atom could attain the helium configuration by gaining one electron. If this change occurred, we would say that hydrogen has a 1- oxidation number. Hydrogen does indeed exhibit a 1- oxidation number in some compounds.
In Group IIA we would expect the loss of the two s electrons for the atom to achieve the same configuration as the prior noble gas. That loss leads to a prediction of 2+ oxidation number for the alkaline earth metals. These two columns exhibit the oxidation numbers predicted for them.
Beginning with Group IIIB, we have atoms in which the highest energy electrons are not in the outer level. For instance, scandium has the configuration ls2 2s2 2p6 3s2 3p6 4s2 3 d1.
Scandium's outer level is the fourth level containing two electrons. Its highest energy electron, however, is the one in the 3d sublevel.
For the transition elements, it is possible to lose not only the outer level electrons, but also some lower level electrons. The transition elements exhibit oxidation numbers varying from 2+ (representing loss of the two outer s electrons) up to 7+.
We would predict scandium to show only 2+ and 3+ oxidation numbers. In actual practice, the element shows only the 3+ oxidation number. Titanium, which has one more 3d electron than scandium, should show 2+, 3+, and 4+, and it does.
As we continue across the fourth row, vanadium has a maximum oxidation number of 5+, chromium 6+, and manganese 7+. Iron, which has the configuraration 1S22S22p63S23p64S23d6, has only 2+ and 3+ oxidation numbers. Recall that an atom with a half-full sublevel represents a particularly stable configuration. To take iron higher than 3+ would mean breaking up a half-full 3d sublevel.
Group IIIA elements lose three electrons and have an oxidation number of 3+. Thallium, in addition to the 3+ oxidation number, exhibits a 1+ oxidation number. If we look at its configuration, we can understand why. The thallium configuration ends 6s2 4f'14 5d1O6p1
The large energy difference between the 6s and the 6p electrons, makes it possible to lose only the 6p electron. That loss leads to an oxidation number of 1+. If stronger reaction methods are used, thallium also has an oxidation number of 3+. For the same reason, tin and lead in Group IVA may have a 2+ or 4+ oxidation number.
In Groups VA, VIA, and VIIA, there is a general tendency to gain electrons to complete the octet. The outside level is already more than half filled. These elements show oxidation numbers of 3- (Group VA), 2- (Group VIA), and 1- (Group VIIA).
It is also possible for these elements to lose electrons and have positive oxidation numbers. The tendency to lose electrons increases as we move down a column. This tendency will be discussed in more detail later.
SUMMARY
1. There have been many attempts to classify the elements in a systematic manner.
2. The modern periodic law states: The properties of the elements are a periodic function of their atomic numbers.
3. Today's periodic table is based on the electron configurations of atoms. All elements in a horizontal line of the table are called a period; all elements in a vertical line are called a group or family.
4. The most stable atoms, the noble gases, have eight electrons in the outer level. Helium atoms are stable with two electrons in the outer level.
5. Full and half-full sublevels represent atoms in states of special stability.
6. Elements with one, two, or three electrons in the outer level tend to be metals. Elements with five, six, seven, or eight outer electrons tend to be nonmetals.
7. The periodic table, together with the octet rule, may be used to predict oxidation numbers.
More on the Periodic Table:
For a PowerPoint presentation Click Here.
.......... The Song of The Elements by Tom Lehrer
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page