Matter
![]()
Matter
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
MATTER
The world around us is filled with objects of many kinds. There are people, chairs, books, trees, lumps of sugar, ice cubes, drinking glasses, doorknobs, and an endless number of other familiar objects.
Each of these objects may be characterized by its size, shape, use, color, and texture. Many unlike objects have certain things in common. For example, a tree and a chair are both made of wood. Millions of other objects with different shapes and purposes may also be made of wood. The word material is used in referring to a specific kind of matter (such as wood). Familiar materials include wood, steel, copper, sugar, salt, nickel, marble, concrete, and milk,
3:1 HETEROGENEOUS MATERlALS
Most of the things we see around us contain two or more different materials. Sometimes it is necessary to use a microscope to distinguish between these different materials. Wood, granite, concrete, and milk are examples. If we look closely at granite, we can see at least three minerals. These minerals are quartz, biotite, and feldspar.
If a piece of granite rock appears to be uniform. Under a microscope, however, we can see particles suspended in water.

Milk is not uniform. Such nonuniform materials are called heterogeneous (het uh roh JEE nee uhs) materials. One type of material can be separated from the other material in milk. Fat globules can be removed by a cream separator.
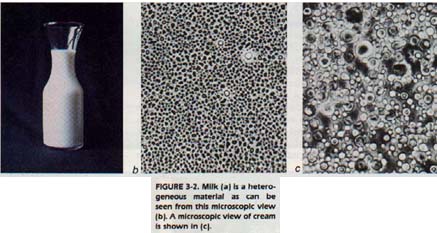
Any physically separate part of a material is called a phase. A phase is any region with a Uniform set of properties. We can distinguish between different phases of the same material.
For example, ice and water are different phases of the same material. All the material in the water region has the same set of properties. Likewise, all the material in the ice region has the same set of properties. Ice and water are different states (solid and liquid) of the same material.
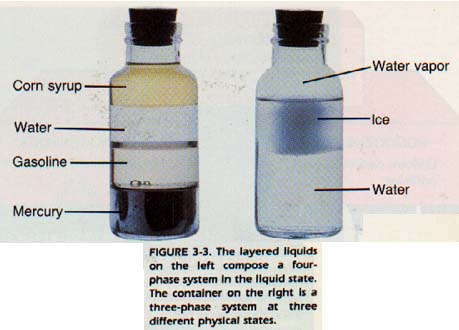
We may now define a heterogeneous material as one that is composed of more than one phase. The different phases in a heterogeneous material are separated from each other by definite boundaries called interfaces. In the two-phase system of ice and water, the surfaces of the ice and water are the interfaces.
3:2 HOMOGENEOUS MATERlALS
Materials which consist of only one phase are called homogeneous (hoh moh JEE nee uhs) materials. Since they are homogeneous, there must be a uniform distribution of the particles within the material.
If you break a piece of homogeneous matter into smaller pieces, each piece will have the same properties as every other small piece. If you look at one of the pieces under a microscope, it is impossible to distinguish one part as being a different material from any other part.
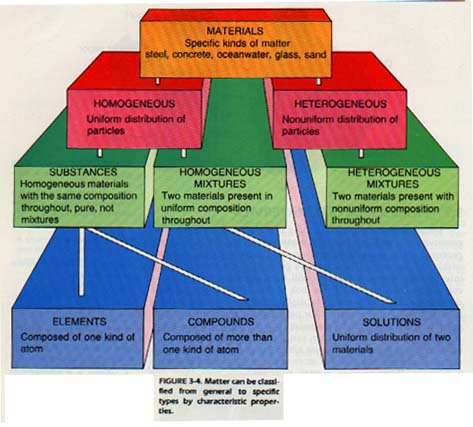
Examples of homogeneous materials are sugar, salt, seawater, quartz, and window glass. Some homogeneous matter can be classified as mixtures. A mixture contains more than one kind of material. Heterogeneous matter is always composed of more than one phase and is always a mixture. Homogeneous matter composed of more than one material is called a solution.
Solutions such as seawater, window glass, and gold-silver alloys vary in composition from sample to sample. If we put a small amount of pure salt into pure water and let it stand, we get a solution, or homogeneous mixture.
If we add a larger amount of pure salt to the same amount of pure water, we again get a solution. The composition of the second sample would differ from the first. The second sample contains more salt in an equal volume of water.
Homogeneous mixtures, or solutions, have variable compositions. For example, we may add 5, 10, or 15 grams of salt to 100 grams of water. In each case, the resulting material is homogeneous. A solution may also be defined as a single phase which can vary in composition. Solutions are not necessarily liquid. Air is a homogeneous material composed of nitrogen, oxygen, and smaller quantities of other gases. Its composition varies from place to place. However, each sample of air is a homogeneous mixture. Different types of window glass have different compositions, yet each type is homogeneous. Both air and glass are solutions.
A solution consists of a solute (dissolved material) in a solvent (dissolving material). In the case of two liquids in solution, the solvent is the component which is the larger proportion of the whole solution.
The solute is scattered in the solvent in very small particles (molecular or smaller). Because of this scattering, the solution appears uniform, even under the most powerful optical microscope. Since the scattering of particles appears to be completely uniform, solutions are classified as homogeneous materials.
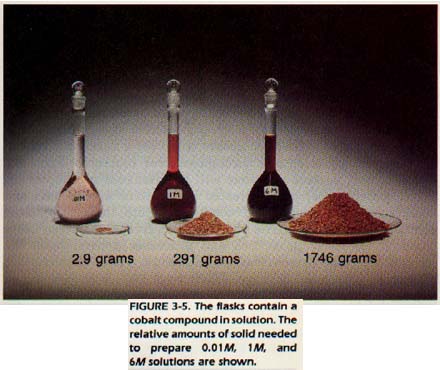
In your laboratory work, you will be using solutions labeled with a number followed by the letter "M." The symbol represents the term molarity. The exact meaning of molarity will be studied in Chapter 5.
In the meantime, you should keep in mind that molarity is used to indicate the amount of solute in a specific amount of solution. A 6M (six molar) solution contains 60 times as much solute as a 0.11M (tenth molar) solution of the same volume.
3:3 SUBSTANCES
Some homogeneous materials such as pure salt, pure sugar, or pure sulfur always have the same composition. Such materials are called substances.
A large part of chemistry is the study of the processes by which substances may be changed into other substances.
According to the atomic theory, matter is made of very tiny particles called atoms.
Substances are divided into two classes. Substances composed of only one kind of atom are called elements. Examples are sulfur, oxygen, hydrogen, nitrogen, copper, gold, and chlorine.
Substances composed of more than one kind of atom are called compounds. The atoms in the particles of compounds are always in definite ratios. Thus, water contains hydrogen atoms and oxygen atoms in a ratio of 2 to 1.
To summarize, all matter can be classified as heterogeneous mixture, solution, compound, or element.
The development of this system of classification played a significant role in the early development of chemistry. Early chemists spent much time and energy sorting the pure substances from the mixtures.
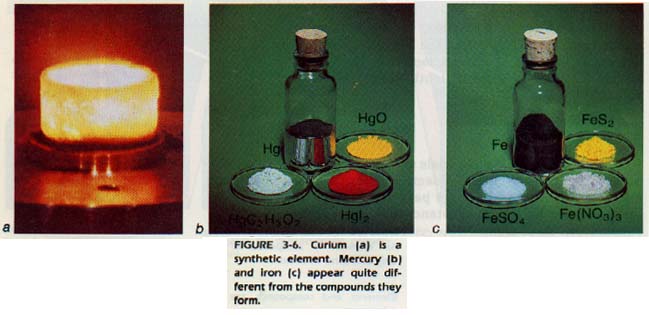
Chemists know of 88 naturally occurring elements. A complete list of the elements is found on page 60. Uranium is the natural element with the most complex atoms. Two elements, technetium and promethium, which have simpler atoms than uranium are not found in nature.
Astatine and francium have been detected in nature. However, they are present in such small amounts that they cannot be easily separated from their ores. These four elements are not normally counted among the natural elements.
These synthetic elements, and over a dozen others, can be produced by scientists through nuclear reactions. Synthetic elements and nuclear reactions will be discussed in Chapter 28.
Chemists today are interested in reactions of elements and compounds, the analysis of compounds into their component elements, and the synthesis of compounds from elements or other compounds. These properties and processes depend upon the structure of the elements and compounds. Thus, chemists are very much interested in these structures.
3:4 PHYSICAL PROPERTIES
The properties of a substance can be divided into two classes. One class depends on the substance itself. The other depends for the most part on the action of the substance in the presence of other substances.
The first class of properties is called physical properties. Length color, and temperature are physical properties.
Physical properties may be divided into groups: extensive properties and intensive properties.
Extensive properties depend upon the amount of matter present. Some of these properties are mass, length, and volume.
Intensive properties do not depend upon the amount of matter present. For example, each sample of a substance, regardless of its size, has the same density throughout.
Other intensive properties include malleability, ductility and conductivity. For example, copper can be hammered quite easily into thin sheets. It is more malleable than iron, which resists this pounding.
Copper can also be drawn out into a fine wire; it is quite ductile. Both copper and silver have high heat and electrical conductivity. A high heat conductivity or electrical conductivity means that a substance offers little resistance to the flow of heat or electricity.

Silver has a high heat conductivity and a silver spoon will become hot if left in a hot pan of soup. In addition to density, the intensive properties most important to the chemist are color, crystalline shape, melting point, boiling point, and refractive index (ability to bend light).
3:5 PHYSlCAL CHANGES
Changes like pounding, pulling, or heating do not change the chemical character of a substance. Pounded copper is still copper. Only its physical appearance is changed. Thus, these changes are called physical changes.
Dividing a piece of material into smaller pieces, tearing paper dissolving sugar in water, and pouring a liquid from one container to another, are other examples of physical changes.
Physical changes occur when a substance melts or boils. At the melting point, a solid changes to a liquid. At the boiling point, a liquid changes to a gas.
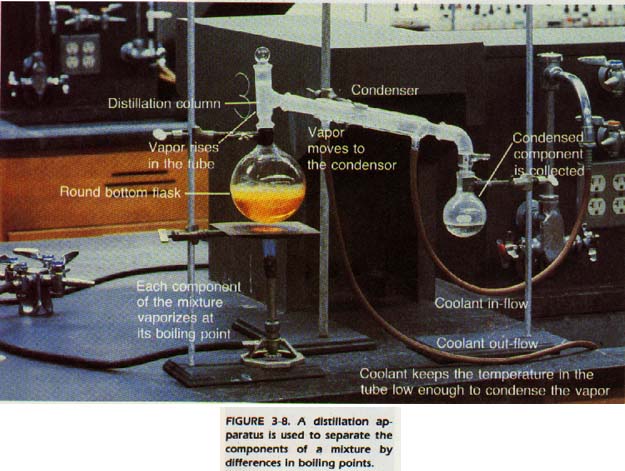
Such physical changes are called changes of state, since the substance is not altered except for its physical state. A knowledge of physical properties and changes can be applied to separating mixtures. Separating substances by distillation is a change-of-state operation.
It is used to separate substances with different boiling points. For instance, you can separate a solution of salt in water by heating the solution to a temperature equal to the boiling point of the solution. The water will then be turned to steam and escape from the container. The salt, whose boiling point has not yet been reached, will remain.
Another separation based on phase difference depends upon the solubility of one substance in another. Most substances have a specific solubility (amount af solute that dissolves) in water at a given temperature. Therefore, it is usually possible to separate two substances in the same solution by a process called fractional crystallization. The insoluble substance which forms from a solution is called a precipitate. This process may be called crystallization since most substances form crystals when they precipitate.
Notice in Figure 3-10 that, at 70oC, potassium bromide (KBr) is less soluble than potassium nitrate (KN03). If a water solution containing equal amounts of both substances is allowed to evaporate at 70oC, the potassium bromide will crystallize first.
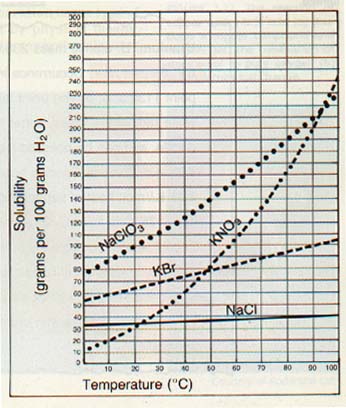
This process is used quite often by chemists to purify laboratory chemicals. It is also used in industry in the production of many crystalline items, such as sugar, salt, and drugs.
Both distillation and crystallization are useful in separating mixtures. As a rule, any mixture can be separated by physical changes. In some cases, however, such separations are not practical.
3:6 CHEMlCAL PROPERTlES
Some properties of matter depend upon the action of substances in the presence of other substances. These properties are called chemical properties.
In order to determine the chemical properties of a substance we must know the kinds of chemical changes that the substance can undergo. Does it burn? Does it help other substances to burn? Does it react with water? Does it react with acids and/or bases? With what other kinds of substances does it react? Such questions help to determine the chemical properties of a substance.

3:7 CHEMlCAL CHANGES
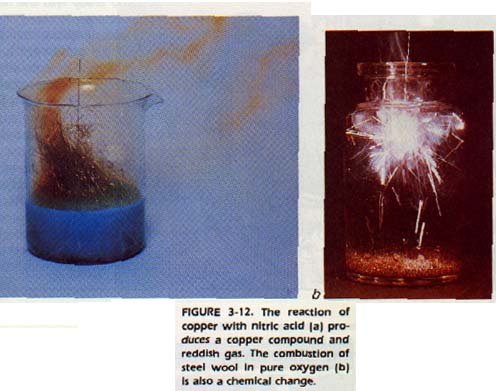
Suppose you are given two test tubes containing colorless liquids. One test tube contains water. The other contains nitric acid. If you place a pinch of sugar in the water, it will disappear and the liquid remains colorless.
The sugar has dissolved and you now have a sugar-water solution. A physical change has occurred. If you put a small piece of copper into the other test tube, it will also disappear. However, the liquid will turn blue and a brown gas will be given off.
You now have a solution of copper nitrate and some nitrogen dioxide gas. A chemical change has occurred.
Let us take another example. Sodium is a silvery, soft metal which reacts violently with water. Chlorine is a greenish yellow gas, which is highly corrosive and poisonous. However, if these two dangerous elements are allowed to combine, they produce a white crystalline solid which neither reacts with water nor is poisonous. It is common table salt, sodium chloride. In this case, the properties of the reactants have disappeared. The new substance formed has different properties.
Whenever a substance undergoes a change so that one or more new substances with different properties are formed, a chemical change (reaction) has taken place. Burning, digesting, and fermenting are examples of chemical changes.
The separation of compounds into their component elements always requires a chemical change. Such a separation is one type of analysis. Mixtures can always be separated by physical means. However, it is sometimes more convenient to separate mixtures by a chemical change.
SUMMARY
1. A phase consists of uniform matter. One phase is separated from other phases by boundaries called interfaces.
2. Heterogeneous matter is made of more than one phase. The phases usually can be separated by physical means.
3. A mixture is a combination of two or more substances which retain their individual properties.
4. Homogeneous matter is made of only one phase.
5. A solution is a homogeneous mixture consisting of a solute dissolved in a solvent. The component parts need not be present in any specific ratios.
6. Compounds are substances made of more than one kind of atom. The component atoms are present in definite ratios.
7. Elements are made of one kind of atom and are the basic substances of the universe. They cannot be broken down into simpler substances by ordinary chemical means.
8. Physical properties depend upon the substance itself.
9. Extensive physical properties, such as mass and length, depend upon the amount of matter present. Intensive properties, such as ductility and melting point, depend on the nature of matter itself
10. A physical change in a substance does not alter its chemical character. A change of state is a physical change from one state-- solid, liquid, or gas to another.
11. The chemical properties of a substance depend upon the action of the substance in the presence of other substances.
12. A chemical change in a substance involves the formation of new substances with different properties. Chemical changes must be used to separate the elements composing a compound.
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
 Return to the Big Chem Page
Return to the Big Chem Page