Chemical Equations
![]()
Chemical Equations
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
CHEMICAL EQUATIONS
You have already seen that chemists have a shorthand method for writing the formulas of substances. They also use this shorthand for describing the changes which substances undergo. Consider the following statement: "Two molecules of ethyne gas will react with five molecules of oxygen gas to produce four molecules of carbon dioxide gas and two molecules of liquid water. How much easier it is to write
2C2H2(g) + 502(g) ---> 4C02(g) + 2H20(1)
to express the burning of ethyne.
6:1 REPRESENTlNG CHEMlCAL CHANGES
The formulas of compounds are used to represent the chemical changes that occur in a chemical reaction. A chemical reaction is the process by which one or more substances are changed into one or more different substances.
A chemical reaction may be represented by an equation. A correct chemical equation shows what changes take place. It also shows the relative amounts of the various elements and compounds that take part in these changes.
The starting substances in a chemical reaction are called reactants. The substances which are formed by the chemical reaction are called products.
Reactants ---> Products
The letters in parentheses indicate the physical state of each substance involved.
The symbol (g) after a formula means that the substance is a gas. Liquids are indicated by the symbol (1), and solids by the symbol (c) or (s). The symbol (c) for solids indicates that the solid is crystalline.
Since many chemical reactions take place in water solution, substance in water solution is shown by the symbol (aq). This symbol comes from the word aqueous (Latin aqua). For example, if a water solution of sulfurous acid is warmed, it decomposes. The products of this reaction are water and sulfur dioxide, a gas.
H2SO3(aq) ---> H20(l) + SO2(g)
2 BALANClNG EQUATlONS
A chemical reaction can be represented by a chemical equation. To write an equation which accurately represents the reaction, we must perform correctly three steps.
Step 1. Determine exactly what are the reactants and the products. For example, when propane gas burns in air, the reactants are propane, C3H8 , and oxygen O2. The products formed are carbon dioxide (CO2 and water (H2O).
Step 2. Assemble the parts of the chemical equation. Write the formulas for the reactants on one side of the equation, usually on the left, and connect them with plus signs. Write the formulas for the products on the other side of the equation. Connect the two sides using an arrow to show the direction of the reaction. Thus,
C3H8 + O2 ---> CO2 + H2O
reactants yield products

The symbols and formulas must be correct. Ah, the summer assignment. If not, Step 3 will be useless. Refer to the Symbols and Valence Study Sheet (if you've not yet completed the summer assignment when attempting to write correct formulas. We will ignore the symbols which indicate physical state while we learn to balance equations.
Step 3. Balance the equation. Balancing means showing an equal number of atoms for each element on both sides of the equation.
Recall that the law of conservation of mass states that the same amount of matter must be present both before and after all chemical reactions. Therefore, the same number and kinds of atoms must be present on both sides of the equation.
Check the preceding equation. It is not balanced. There are three carbon atoms on the left, but only one carbon atom on the right. To put the carbon in balance, we place the coefficient 3 before the carbon dioxide on the right.
In balancing an equation, change only the coefficients. Never change the subscripts! To do so would change the substance represented.
......... Print Equations & Answers Practice Sheet.
TYPES OF CHEMICAL REACTIONS:
Synthesis. In a synthesis reaction, two elements combine to form a compound.
Decomposition. In a decomposition reaction, a compound is broken down into it's elements.
Single Displacement. In a single displacement reaction, an element replaces another element from a compound.
Double Displacement. In a double displacement reaction, two compounds exchange partners to form two new compounds.
......... Get the Notes for Chapter 6 for more information.
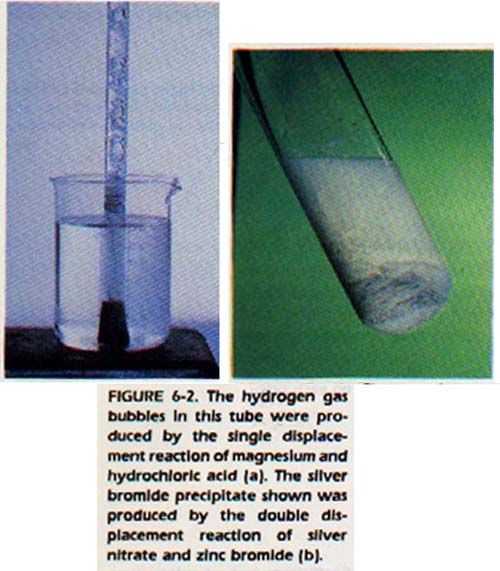
SUMMARY
1. Chemists use equations to describe the changes which substances undergo.
2. The physical state of substances in equations is shown by (g) for gas, (1) for liquid, (c) or (s) for solid, and (aq) for a water solution.
3. A chemical reaction is the process by which one or more substances are changed into one or more new substances.
4. Reactants are the starting substances in a reaction. Products are the substances resulting from a reaction.
5. A chemical equation represents changes that take place in a reaction. It also shows relative amounts of reactants and products.
6. Balancing an equation means adjusting coefficients so that there are the same number of atoms of each element on both sides of the equation.
7. In a single displacement reaction, one element displaces another element. In a double displacement reaction, ions from two compounds are interchanged.
8. In a decomposition reaction, a compound breaks down into two or more simpler substances.
9. In a synthesis reaction, two or more substances combine to form a more complex substance.
Ah Yaz Indeed!
......... Print Equations & Answers Practice Sheet.
For a PowerPoint presentation Click Here.
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page