Molecular Structure
![]()
Molecular Structure
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
MOLECULAR STRUCTURE
There are several ways of looking at the structure of molecules order to describe their shape. We will consider four of these:
The first of these ideas takes into account the repulsive of electron pairs surrounding an atom.
The second method different ways in which atomic orbitals can combine to orbitals surrounding more than one nucleus. The electrons occupying these combined orbitals then serve to bind the atoms.
The third method considers molecules with more than one possible structure.
The fourth method considers orbitals for molecules as a whole instead of atoms.
In order to describe the shape of a molecule or polyatomic ion, it is useful to draw an electron dot diagram for it.
Consider the water molecule. It is composed of one oxygen and two hydrogen atoms. All electrons are identical. We use different symbols only to help us understand how we arrive at the final structure by combining an oxygen and two hydrogens, we obtain the electron dot diagram.
![]()
It the only arrangement of electrons in which all three atoms achieve a full outer level. Note that two pairs of electrons in the outer level of oxygen are involved in bonding the hydrogens are called shared pairs. The other two pairs of electrons are not involved in bonding. They are called unshared pairs.
13:1 METHOD 1: PAlR REPULSlON
One way of looking at molecules is to consider electron repulsion. Each bond and each unshared pair in the outer level of atom form a charge cloud which repels all other charge clouds. In part, this repulsion is due to all electrons having the same charge.
Another more important factor is the Pauli exclusion principle. Although electrons of opposite spin may occupy same volume of space, electrons of the same spin may not do so. The repulsions resulting from the Pauli principle are greater than the electrostatic ones at small distances. Because of these repulsions, atoms cannot be compressed.
The repulsions between the charge clouds in the outer level of atoms determine the arrangement of the orbitals. The orbital arrangement, in turn, determines the shape of molecules.
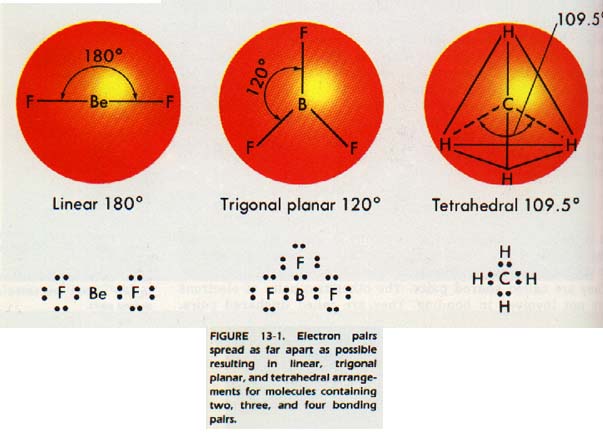
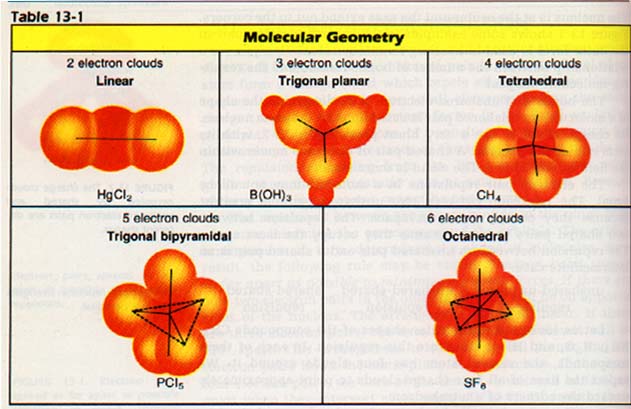
As result, the following rule may be stated: Electron pairs spread as far apart as possible to minimize repulsive forces. If there only two electron pairs in the outer level, they will be on opposite sides of the nucleus. The arrangement is called linear.
If are three electron pairs, the axes of their charge clouds will 120o apart. This arrangement is called trigonal planar and electron pairs lie in the same plane as the nucleus.
If there four electron pairs, the axes of the charge clouds will be farthest apart when they intersect at an angle of 109.5o.
Of course, axes will not all lie in the same plane. The easiest way to them is to imagine them is to imagine a regular tetrahedron. A tetrahedron is a figure having four faces, each of which is an equilateral triangle. The nucleus is at the center and the axes extend out to the corners. 13-1 shows some examples in which each electron pair in outer level is used in bonding to another atom. Can you see a between the number of bonds formed and the molecular shapes?
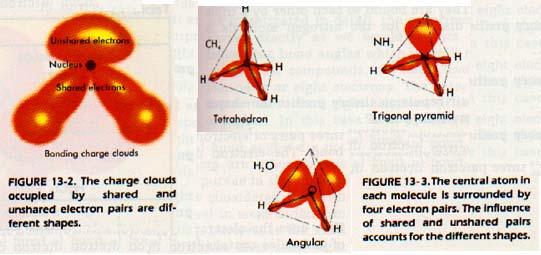
The bonds and unshared electron pairs determine the shape a molecule. An unshared pair is acted upon by only one nucleus. It's charge cloud is like a very blunt pear, Figure 13-2, with its stem end at the nucleus. A shared pair of electrons moves within field of two nuclei. The cloud is more slender.
The electron pair repulsions in a molecule may not all be equal. The repulsion between two unshared pairs is greatest when they occupy the most space. The repulsion between shared pairs is least because they occupy the least space. The repulsion between an unshared pair and a shared pair is an intermediate case.
unshared-unshared repulsion > unshared-shared repulsion > shared-shared repulsion
Electron pair repulsion strengths may not bc equal.
Let us look at the molecular shapes of the compounds CH4, ,H2O, and HF to illustrate this repulsion. In each of these compounds, the central atom has four clouds around it. We expect the axes of all four charge clouds to point approximately to the corners of a tetrahedron.
More Molecular Geometry
And Still more Molecular Shapes
13:3 SlGMA AND PI BONDS
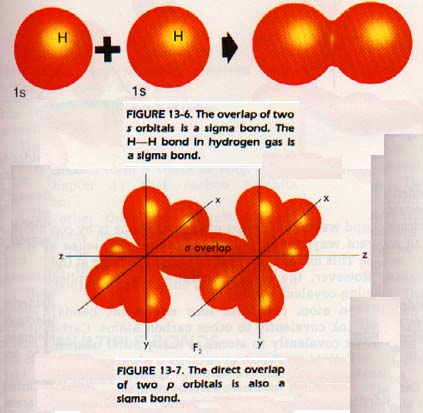
A covalent bond is formed when an orbital of one atom overlaps an orbital of another atom and they share the electron pair the bond. For example, a bond may be formed by the overlap two s orbitals. A bond formed by the direct overlap of two orbitals is called a sigma bond, σ, and is designated s. A sigma bond is also formed by the overlap of an s orbital of one atom with a p orbital of another atom.
When two p orbitals overlap, there are two possibilities since p orbitals are not spherical. If two p orbitals overlap along an axis in an end-to-end fashion a sigma type (a) bond is formed as shown in Figure 13-7. If, however, the two p orbitals overlap sideways with their axes parallel, as in Figure 13-8, they form what is called a pi, π, bond.
13:4 METHOD 2: HYBRlD ORBlTALS
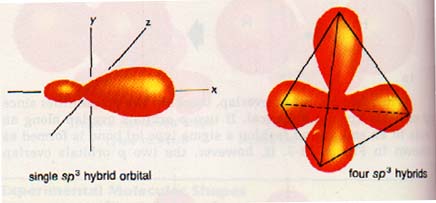
We would expect the carbon atom with four outer to have two p orbitals available for bonding. However, it has been discovered that carbon does not usually form two p orbital Instead, according to theory, the s and p orbitals of carbon merge to form four equivalent hybrid orbitals. If one s and three p orbitals merge, four sp3 hybrid orbitals are formed. The orbitals are arranged in tetrahedral fashion.
SUMMARY
1. The shape of molecules can be approximated by assuming the mutual exclusion of electron pairs. This repulsion of electron pairs has two sources. All electrons have the same electrostatic charge and are subject to the Pauli exclusions principle. Electron pairs spread as far apart as possible.
2. The shape of a molecule containing three or more atoms is determined by the number of electron clouds in the outer level.
3. If the central atom has two bonds and no unshared pairs, the molecule is linear.
4. If there are three bonds and no unshared pairs, the molecule is trigonal planar.
5. If there are four bonds, the molecule is tetrahedral.
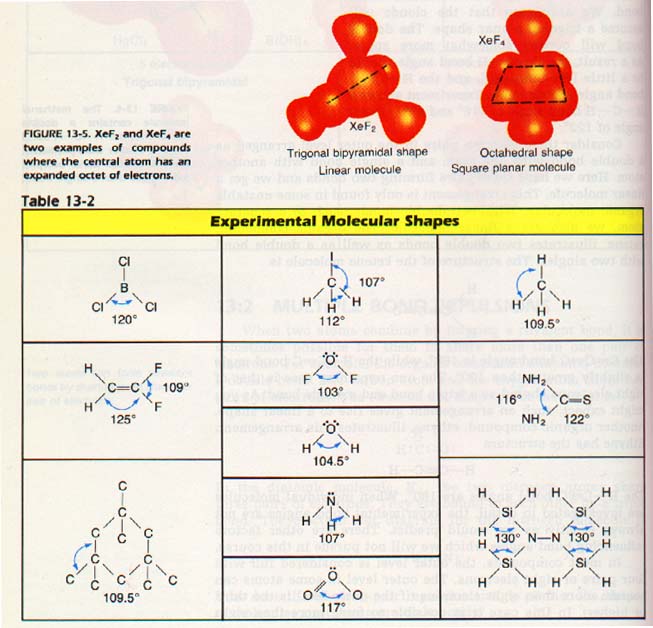
6. The actual bond angles may vary from predicted angles. Unshared electron pairs occupy the most space. Shared pairs occupy the least space.
7. Two atoms sometimes share more than one pair of electrons forming double and triple bonds. This possibility must be considered when discussing molecular geometry.
8. Molecular covalent bonds are formed by overlap of atomic orbitals: s-s, s-p, and p-p.
9. If two s orbitals or s and p orbitals overlap, a sigma bond, σ, is formed. If two p orbitals overlap end-to-end, a pi, π, bond is formed.
10. s and p orbitals of the same atom can combine to form hybrid orbitals. One s and three p orbitals merge to form four tetrahedral sp3 hybrids. One s and two p orbitals merge to form three planar sp2 hybrid orbitals. One s and one p orbitals merge to form two linear sp hybrid orbitals.
11. A double bond is formed by the sigma, σ, overlap of two sp2 orbitals and the pi, π, overlap of the two p orbitals.
12. A triple bond is formed by the sigma, σ, overlap of two sp orbitals and the pi, π, overlap of four p orbitals.
More on Molecular Structure:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page