Chemical Formulas
![]()
Chemical Formulas
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
CHEMlCAL FORMULAS
Although there are only a few more than 100 elements, there are literally millions of compounds known to chemists. It is convenient to represent elements and compounds by the use of symbols. These representations of substances are another way to help us to classify these substances quickly. This ability to classify simplifies the study of the vast number of substances with which chemists work.
4: 1 SYMBOLS
The chemical symbols of the elements are a form of shorthand. They take the place of the complete names of the elements. A symbol may represent one atom of an element. Scientists throughout the world have agreed to represent one atom of aluminum by the symbol Al.
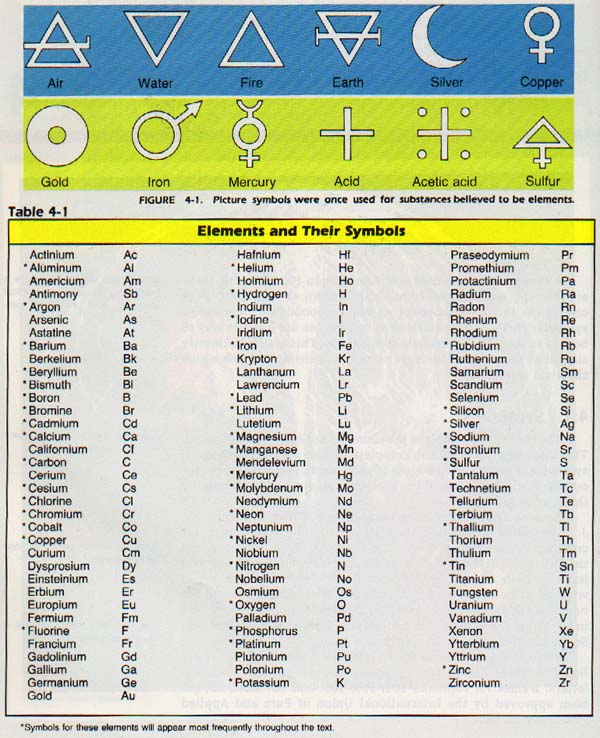
Ancient symbols for some elements are shown in Figure 4-1. J. J. Berzelius, a Swedish chemist, is generally given credit for creating the modern symbols for elements.
Berzelius proposed that all elements be given a symbol corresponding to the first letter of their names. In the case of two elements which began with the same letter, a second letter or a letter outstanding in the name was added. In some cases, the Latin name of the element was used. Thus, the symbol for sulfur is S; selenium, Se; strontium, Sr; and sodium, Na (Latin natrium = sodium).
The symbols that have been agreed upon for 103 elements are listed in Table 4-1. Notice that they are capital and lowercase letters. Names for elements 104, 105, 106, and 107 have not yet been approved by the International Union of Pure and Applied Chemistry (IUPAC).
4:2 CHEMlCAL FORMULAS
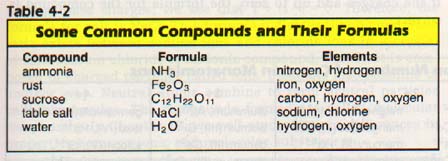
Chemists also use combinations of symbols to represent compounds. Compounds are substances in which two or more elements are chemically combined. Compounds are represented by chemical formulas.
A chemical formula is a combination of symbols which represents the composition of a compound. Formulas often contain numerals to indicate the proportions in which the elements occur within a compound. For example, we have learned from experiments that water is composed of the elements hydrogen and oxygen. We also know that two atoms of hydrogen will react (combine chemically) with one atom of oxygen to form one molecule of water.
As a formula for water, we write "H20." The small subscript, 2, after the H indicates that there are two atoms of hydrogen in one molecule of water. Note that there is no subscript after the oxygen. If a symbol for an element has no subscript, it is understood that only one atom of that element is present.
A formula shows two things. It indicates the elements present in the compound and the relative number of atoms of each element in the compound. How many atoms of each element are present in each compound in Table 4-21
Most compounds containing the element carbon are classed as organic compounds. Formulas for organic compounds are written according to a different set of rules. These rules will be studied in detail in Chapter 29.
4:3 OXlDATlON NUMBER
Through experiments, chemists have determined the ratios in which most elements combine. They have also learned that these combining ratios depend upon the structure of their atoms. This relationship will be explored fully in Chapters 8-10.
In the meantime, you should know that the atoms can acquire an electric charge. They may also attach themselves to other atoms so that the entire group acquires an electric charge. Such charged atoms are called ions. An ion made of more than one atom, for example OH-, is called a polyatomic ion.
When a single atom acquires a charge, that charge is known as its oxidation number. Table 4-3 lists the oxidation numbers of some common elements.
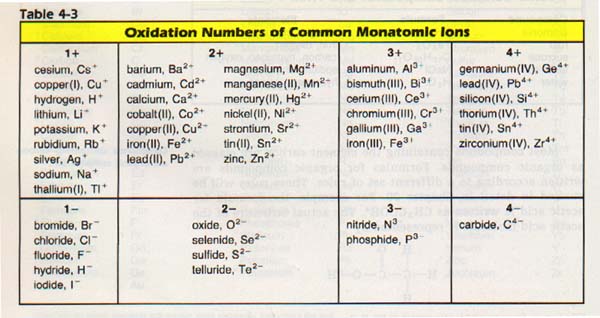
Table 4-4 lists the charges of some common polyatomic ions.
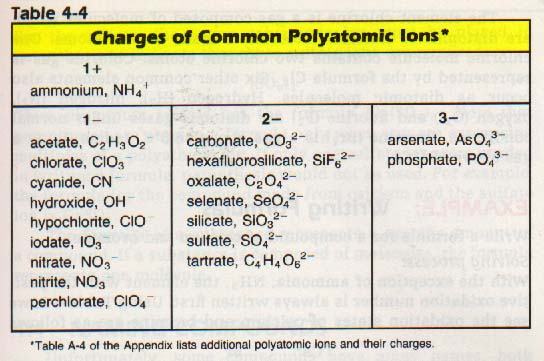
We may use this information to write correct chemical formulas. Atoms and ions combine chemically in definite ratios. Oxidation numbers of elements and the charges on polyatomic ions tell us these combining ratios. The way to determine the ratio of elements in a compound is to add the charges algebraically. If the charges add up to zero, the formula for the compound is correct.
Common table salt NaCl is made from sodium, Na, and chlorine, C1. Table 4-3 shows a 1+ charge for sodium ions and a 1- charge for chloride ions.
Na+C1-
Adding these charges, we see that 1 + (1-) = O. Therefore, the formula for salt is NaC1. This formula indicates that a one-to-one ratio exists between sodium ions and chloride ions in a crystal of salt.
Sodium chloride is an ionic compound, that is, it is composed of charged particles called ions. Elements also combine in another way. Neutral atoms combine to form neutral particles called molecules. The compounds formed are called molecular compounds. In Chapter 12, we will study distinct differences in properties between ionic and molecular substances.

The element chlorine is a gas composed of molecules which are diatomic. A diatomic molecule is made of two atoms. One chlorine molecule contains two chlorine atoms.
Chlorine gas is represented by the formula Cl2. Six other common elements also occur as diatomic molecules. Hydrogen (H2), nitrogen (N2), Oxygen (02), and fluorine (F2) are diatomic gases under normal conditions. Bromine (Br2) is a gas above 58.8oC. Iodine (I2) is a gas above 184oC.
EXAMPLE: Writing Formulas
Write a formula for a compound of calcium and bromine.
Solving process:
With the exception of ammonia, NH3, the element with the positive oxidation number is always written first. Using Table 4-3, we see the oxidation states of calcium and bromine are as follows
Ca2+ and Br-
Since the sum of the charges must equal zero, two Br- are needed to balance Ca2+ . The correct formula is CaBr2.
Write the formula for a compound made from aluminum and sulfur atoms.
Solving process:
Using Table 4-3, we see the oxidation states of aluminum and sulfur are as follows:
A13+ and S2-
To make the sum of the charge equal zero, we must find the least common multiple of 3 and 2. The least common multiple is 6. Thus, two A13+ and three S2- must combine for the charges to equal zero. The formula is written A2S3.
Write the formula for a compound made from aluminum and the sulfate ion.
Solving process:
Using Tables 4-3 and 4-4, we see the oxidation states of aluminum and the sulfate ion are as follows:
A13+ and S042-
It is necessary to have two A13+ and three S042- in the compound to maintain neutrality. Writing aluminum ions in the formula is simple.
Al2
For the sulfate, the entire polyatomic ion must be placed in parenthesis to indicate that three sulfate ions are required.
(S04)3
Thus, aluminum sulfate has the formula A12(S04)3 . Note that parentheses are used in a formula only when you are expressing multiples of a polyatomic ion.
If only one sulfate ion were needed in writing a formula, parenthesis would not be used. For example, the formula for the compound made from calcium and the sulfate ion is CaS04.
The formula of a substance represents a specific amount of a compound. If a substance is composed of molecules, the formula represents one molecule.
4:4 NAMlNG COMPOUNDS
Unfortunately, some compounds have many names, both common and chemical. However, there is a systematic method of naming practically all the compounds which we will use. The names of only a few compounds, particularly acids, will not be included in this system.
Compounds containing only two elements are called binary compounds. To name a binary compound, first write the name of the element having a positive charge. Then add the name of the negative element. The name of the negative element must be modified to end in -ide. For example, the compound formed by aluminum [A13+] and nitrogen [N3-], With the formula A1N, is named aluminum nitride.
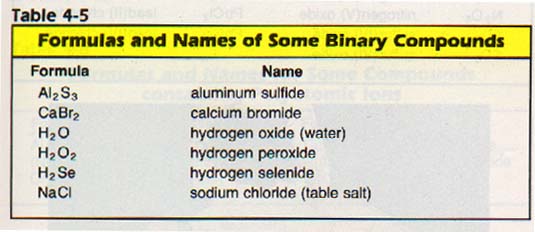
In looking at Table 4-3 we see that some elements have more than one possible charge. Therefore, they may form more than one compound with another element. For instance, nitrogen and oxygen form five different binary compounds with each other!
We must have a way of distinguishing the names of these compounds.
We tell the difference by writing the oxidation number of the element having positive charge after the name of that element. Roman numerals in parentheses are used. Examples of some compounds named according to this system are listed in Table 4-6.
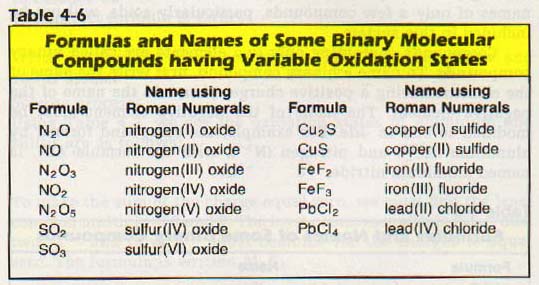
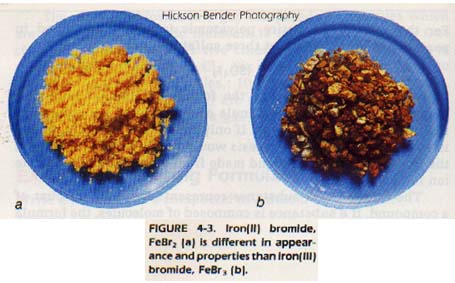
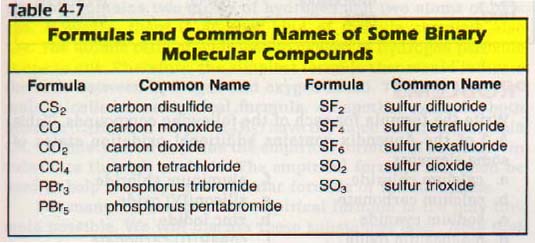
There are many compounds that have been named by an older system in which prefixes indicate the number of atoms present. These names have been in use for so long that these more common names are usually used. Examples are listed in Table 4-7.
Not all compounds ending in -ide are binary. A few negative polyatomic ions have names ending in -ide. Examples are OH (hydroxide), NH2- (amide), N2H2- (hydrazine), and CN- (cyanide). For naming compounds containing more than two elements, several rules apply. The simplest of these compounds are formed from one element and a polyatomic ion. These compounds are named in the same way as binary compounds. However, the ending of the polyatomic ion is not changed. An example is A1P04, which is named aluminum phosphate. Other examples are listed in Table 4-8.

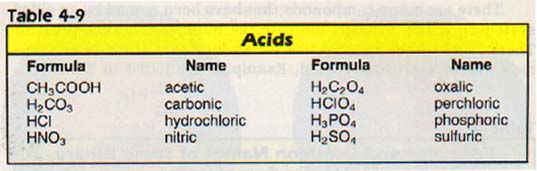
Other rules for naming compounds and writing formulas will be discussed when the need arises. The names of the common acids, for example, do not normally follow these rules. Table 4-9 lists names and formulas for acids which you should memorize.
4:5 MOLECULAR AND EMPlRlCAL FORMULAS
The formulas for compounds that exist as molecules are called molecular formulas. For instance, one compound of hydrogen and oxygen is hydrogen peroxide (H2O2). The formula H202 is a molecular formula because one molecule of hydrogen peroxide contains two atoms of hydrogen and two atoms of oxygen.
However, there is another kind of formula chemists also use. The atomic ratio of hydrogen to oxygen in hydrogen peroxide is one to one. Therefore, the simplest formula that would indicate the ratio between hydrogen and oxygen is HO.
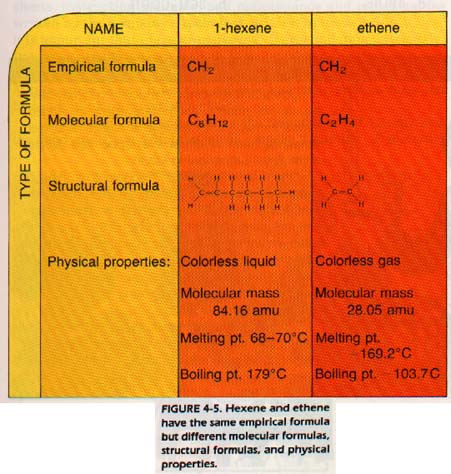
This simplest formula is called an empirical formula. As another example, both benzene (C6H6) and ethyne (C6H6 have the same empirical formula CH.
Chemists can determine the empirical formula of an unknown substance through analysis. The empirical formula may then be used to help identify the molecular formula of the substance. For many substances, the empirical formula is the only formula possible. We will discuss these substances later. Note that the molecular formula of the compound is always some whole number multiple of the empirical formula. The actual calculation of empirical formulas will be covered in Chapter 5.
4:6 COEFFlClENTS
The formula of a compound represents a definite amount of that compound. This amount may be called a formula unit. It may be one molecule or the smallest number of particles giving the true proportions of the elements in the compound. One molecule of water is represented by H2O.
How do we represent two molecules of water? We use the same system as we would use in mathematics: coefficients. When we wish to represent two x, we write 2x. When we wish to represent two molecules of water we write 2H20. Three sodium ions combined with three chloride ions is represented as 3NaCI. We will discuss the use of coefficients in Chapter 6.
SUMMARY
1. A chemical symbol for an element represents one atom of that element when it appears in a formula.
2. A chemical formula is a statement in chemical symbols of the composition of one formula unit of a compound. A subscript in a formula represents the relative number of atoms of an element in the compound.
3. A Polyatomic ion is a stable, charged group of atoms. 4. The combining capacity of an atom or polyatomic ion is indicated by its oxidation number or charge.
5. In chemical compounds, atoms combine in definite ratios. Their combined charges add to zero.
6. A binary compound is cornposed of two elements. Its name is the name of the positive element followed by the name of the negative element modified to end in -ide
7. Some elements can have more than one possible oxidation number. A compound containing such an element is named by showing the oxidation number in Roman numerals in parentheses after the element.
8. A compound formed from one element and one polyatomic ion is named in the same way as a binary compound. However, the ending of the name of the polyatomic ion is not changed.
9. An empirical formula represents the simplest whole number ratio between atoms in a compound.
10. A molecular formula shows the actual number of each kind of atom in one molecule of a compound. It is always a whole-number multiple of the empirical formula.
11. A formula unit represents a definite amount of a compound.
12. The coefficient of a formula indicates the number of molecules or the number of formula units of a nonmolecular substance.
SUMMARY
1. A chemical symbol for an element represents one atom of that element when it appears in a formula.
2. A chemical formula is a statement in chemical symbols of the composition of one formula unit of a compound. A subscript in a formula represents the relative number of atoms of an element in the compound.
3. A polyatomic ion is a stable, charged group of atoms.
4. The combining capacity of an atom or polyatomic ion is indicated by its oxidation number or charge.
5. In chemical compounds, atoms combine in definite ratios. Their combined charges add to zero.
6. A binary compound is composed of two elements. Its name is the name of the positive element followed by the name of the negative element modified to end in -ide.
7. Some elements can have more than one possible oxidation number. A compound containing such an element is named by showing the oxidation number in Roman numerals in parentheses after the element.
8. A compound formed from one element and one polyatomic ion is named in the same way as a binary compound. However, the ending of the name of the polyatomic ion is not changed.
9. An empirical formula represents the simplest whole number ratio between atoms in a compound.
10. A molecular formula shows the actual number of each kind of atom in one molecule of a compound. It is always a whole-number multiple of the empirical formula.
11. A formula unit represents a definite amount of a compound.
12. The coefficient of a formula indicates the number of molecules or the number of formula units of a nonmolecular substance.
.......... Click here to print the Chemical Formulas Assignment and Practice Sheet.pdf.
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() Big Time Extra Help for Big Chem!
Big Time Extra Help for Big Chem!
 Return to the Big Chem Page
Return to the Big Chem Page