Energy and Disorder
![]()
Energy and Disorder
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
ENERGY AND DlSORDER
In the last several chapters, we have been studying macroscopic amounts of matter. We have been interested in physical properties and physical changes. Where chemical reactions were involved, we looked at the quantitative relationships among the reactants and products. Now, we will consider what makes reactions occur.
We have noted that reactions which are exothermic generally take place spontaneously (without help). On the other hand, those that are endothermic are generally not spontaneous.
In everyday life, we can see that changes in nature are usually "downhill." That is, nature tends to go from a state of higher energy to one of lower energy.
Also, natural processes tend to go from an orderly state to a disorderly one.
However, there are some exceptions. How is it possible for products to form which are at a higher energy or a more ordered state than the reactants? In this chapter we will find the answer to such questions. However, keep in mind that unless otherwise stated we will be concerned with reactions taking place at constant temperature and pressure.
Reactions taking place at a constant temperature are isothermal processes. Reactions that take place at constant pressure are isobaric processes.
20:1 ENTHALPY
In Chapter 7, the term enthalpy (H) was defined as heat content. Chemists often work with the change in the enthalpy of a system.
The change in enthalpy or heat content is given the symbol ΔH. In an exothermic reaction, the products have less heat content than the reactants. ΔH is therefore negative. In an endothermic reaction, (ΔH is positive.
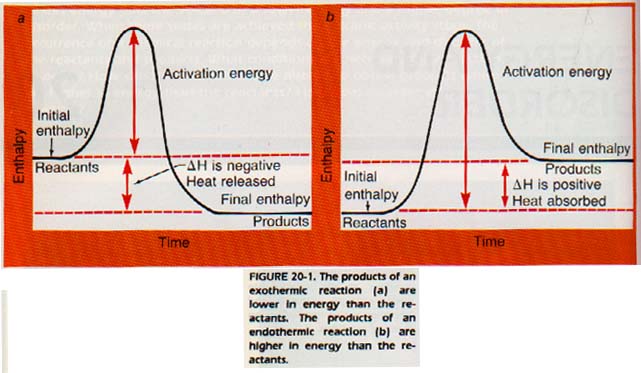
20:2 ENTROPY
Highly exothermic reactions tend to take place spontaneously, However, weak exothermic and endothermic reactions can occur spontaneously. Sometimes these reactions will under stronger reaction conditions such as a temperature increase. Consider the reaction of steam on very hot carbon to form carbon monoxide and hydrogen.
C(c) + H2O(g) + energy ---> H2(g) + CO(g)
The products have a higher heat content than the reactants; Therefore, since heat energy is absorbed in this process, ΔH is positive. It has been determined experimentally that if 1 mole of carbon reacts with 1 mole of steam then, ΔH = 131 kJ.
We have observed in previous sections that most spontaneous reactions seem to have negative ΔH. Since ΔH is positive in this reaction, some additional factor must be at work.
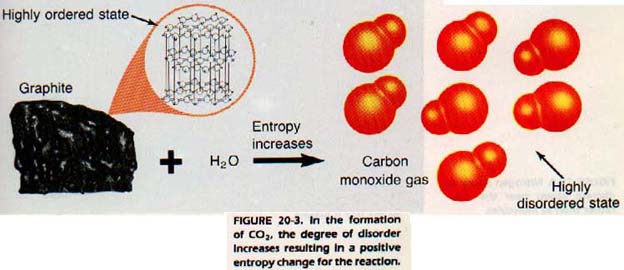
This additional factor is the degree of disorder or entropy. We have seen in Chapter 16 that there is a very orderly arrangement of atoms in crystalline solids. In liquids, there is somewhat less order. Gases lack any orderly arrangement.
The degree of disorder, or entropy, is represented by the symbol S. Change in entropy is symbolized as ΔS. A positive value for ΔS means an increase in the degree of disorder.
That is to say, the system becomes less ordered. Such a change, positive ΔS, occurs when a solid is converted to a liquid or a gas. When the opposite reaction occurs (liquid or gas is converted to a solid), ΔS is negative.

20:3 FREE ENERGY
The combination of H and S is called free energy. It is represented by G; thus, ΔG represents the change in free energy. These quantities are defined by the following relationships.
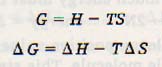
where T is the temperature in kelvin (absolute temperature). It can be shown, both by theory and by experiment, that in a spontaneous change, ΔG is always negative. If a reaction takes place at low temperatures and involves little change in entropy, then the term TΔS will be negligible.
In such a reaction ΔG is largely a function of ΔH, the change in enthalpy.
Thus, most reactions occurring at room temperature have a negative ΔH. Highly endothermic reactions can occur only if TΔS is large. Thus, the temperature is high, or there is a large increase in entropy.
In the endothermic reaction of carbon with steam, both of these conditions occur. ΔS is positive because the orderly arrangement of carbon in the solid is converted to the disorderly arrangement in CO gas. T is high because the reaction only place at red heat (600-900oC) or higher.
If the temperature increases, the reaction stops and goes into reverse. If ΔH and ΔS have the same sign, there will be some temperature at which ΔH and TΔS will be numerically equal, and ΔG be exactly zero.
This state is the thermodynamic definition of system in equilibrium. At equilibrium, the value of the energy G, not ΔG, is at a minimum for the system. All spontaneous processes proceed toward equilibrium. example, a ball rolls down a hill and not up. The bottom of hill is where the ball has the least potential energy. potential energy, technically called free energy, is least when system is at equilibrium.
An interesting result of the entropy contribution to the energy equation is that molecules like H2, 02, and N2, are stable on earth, do not exist on the sun and stars. To see they do not consider the case of N2. In order to decompose mole of N2 molecules, much energy must be supplied.
N2 + energy ---> 2N
energy = ΔH = + 941 kJ
Since ΔH has such a large positive value, at ordinary temperatures N2 is a very stable molecule. This stability is a direct result of TΔS being small in comparison to the large positive ΔH ( makes (ΔG positive).
A gas composed of separate atoms of nitrogen has a greater entropy than one made of N2 molecules. The pairing of nitrogen atoms is a kind of order. Therefore, the decomposition of these molecules represents an increase in entropy (a positive ΔS).
If N2 molecules are exposed to higher temperatures (like those near the sun), the value of TΔS is greater than 941 kJ thus ΔG is negative. As a result, nitrogen exists near the sun as discrete atoms.

20:4 STANDARD STATES
All three quantities (enthalpy, entropy, and free energy) depend on temperature. Thus, chemists have had to agree on a standard set of conditions for measuring these quantities. The conditions chosen are 273 K and 101 kPa. The pressure is specified because the quantities also depend on pressure in some reactions.
20:5 CALCULATlONS ON FREE ENERGY
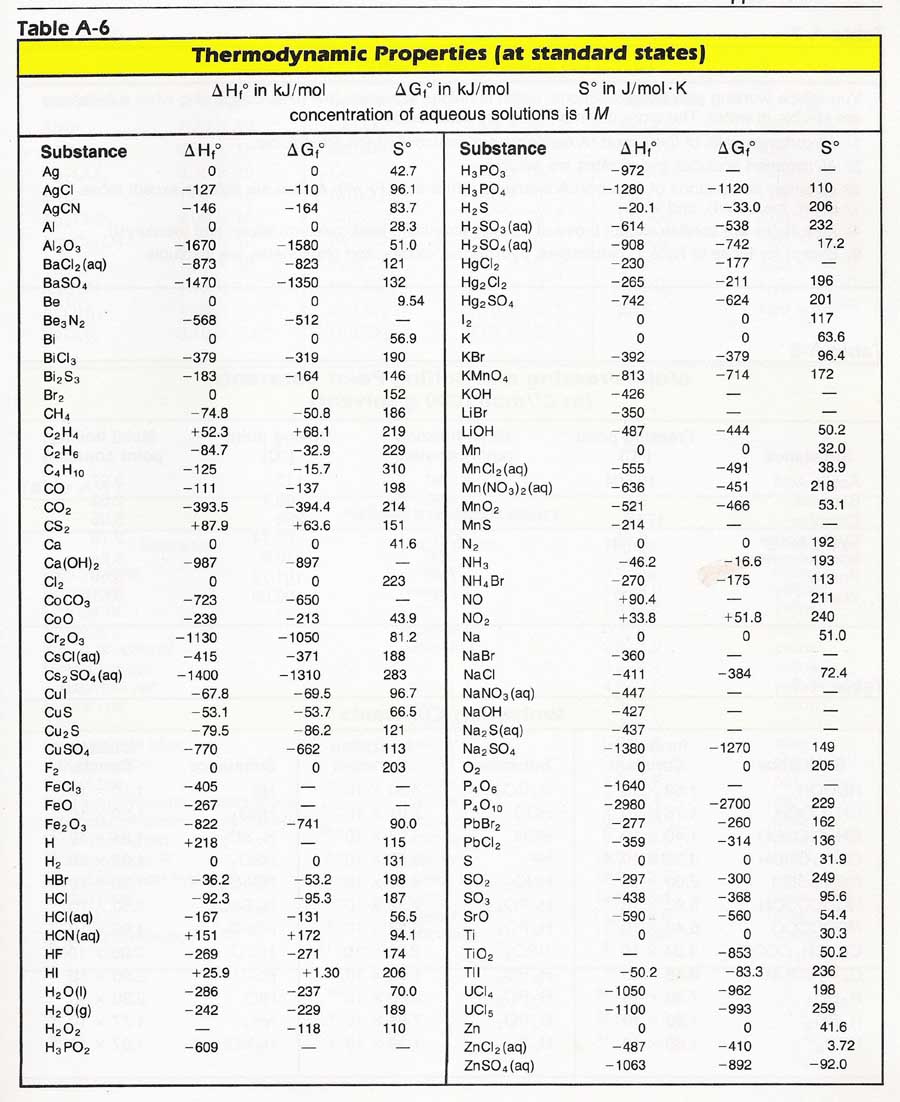
Appendix A-6 lists the standard free energies of formation, ΔGfo), enthalpies of formation ΔHfo), and entropies (So) of some substances. The superscript "o"shows these values have been obtained for standard conditions. The subscript "f" shows they are values for the formation of one mole of the compound from the elements. We already know from Chapter 7 that the enthalpy change for a reaction is found by
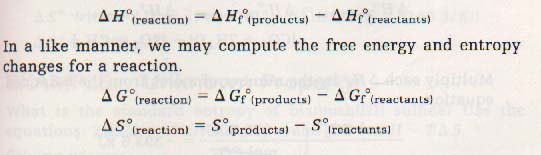
SUMMARY
1. Enthalpy is the heat content in a system.
2. Entropy is the degree of disorder of a system.
3. When the enthalpy change and entropy change differ, the net effect is found from the equation ΔG = ΔH - TΔS. In this equation G is free energy, H is enthalpy, T is the absolute temperature, and S is the entropy.
4. Spontaneous changes have negative free energy changes.
5. When ΔG is zero, the system is at equilibrium.
6. The change in a thermodynamic quantity, ΔH, ΔG, or TΔS, for a reaction is the sum of the quantities of the products less the sum of the quantities of the reactants.
7. The change in the internal energy of a system is the heat absorbed from the surroundings less the work done on the surroundings, ΔE = q - w.
8, The thermodynamic quantities ΔG, ΔH, ΔS, and ΔE are "state" quantities. That is, these quantities depend only on the conditions in one state compared to another. The change is independent of the path chosen in going from one state to another.
9. The change in enthalpy is related to the internal energy by the equation: ΔH = ΔE + Δ(PV).
10. For a constant pressure process, ΔH = q.
More on Entropy:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page