Colligative & Colloidal Properties
![]()
Colligative & Colloidal Properties
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
COLLlGATlVE AND COLLOlDAL PROPERTlES
When a solute is dissolved in a solvent, there is a change in certain properties of the solvent. On the other hand, when particles too large to dissolve are dispersed throughout a liquid, the solvent's properties remain unchanged.
In this chapter, we want to investigate those properties of solvents which are changed by solutes. We also want to look at the behavior of those particles which are too large to dissolve and have no effect on the liquid.
Throughout the first six sections of this chapter, we will be dealing with an ideal solution. In Chapter 18, we were able to define an ideal gas. Unfortunately, we cannot define an ideal solution completely at this point. For the time being, we will just say that the particles of solute in an ideal solution have no effect on each other.
22:l RAOULT'S LAW
Colligative properties are determined by the number of particles in solution rather than by the type of particle in solution. The properties so affected are vapor pressure, freezing point, boiling point, and the rate of diffusion through a membrane.
Consider a solute dissolved in a liquid solvent. Some of the solute particles take up space on the liquid surface normally occupied by solvent particles. These solute particles decrease the opportunity for solvent particles to escape (evaporate) from a liquid surface.
Colligative properties depend on the number of particles in solution:
1. vapor pressure, 2. freezing point, 3. boiling polnt, 4. rate of diffusion through a membrane.
The lowering of the vapor pressure of the solvent varies directly as the mole fraction of dissolved solute. Any nonvolatile solute at a specific concentration lowers the vapor pressure of a solvent by an amount which is characteristic of that solvent. The characteristics of the solute are not involved.
Ionic compounds and molecular compounds with high melting points are typical nonvolatile solutes. To determine the vapor pressure of a solution, we must correct the vapor pressure of the pure solvent for the presence of the solute. The equation used for correcting the vapor pressure is
vapor pressure (solvent) = vapor pressure (solvent) x molefraction (solvent)
This expression is a mathematical statement of Raoult's Law named for Francis Raoult, a French chemist. He first stated the principle that the vapor pressure of a solution varies directly as the mole fraction of solvent.
Raoult's law holds in an ideal solution. We can now define an ideal solution as one in which all intermolecular attractions are the same. In other words, solute-solute, solvent-solvent, and solute-solvent attractions are all essentially the same.
In the case of a volatile solute, Raoult's law is often inadequate to predict the behavior of the solution. However, there are many solutions whose behavior approaches the ideal closely enough to be treated as such. Each volatile component of an ideal solution has a vapor pressure which can be determined by Raoult's law.
22:2 FRACTIONAL DISTILLATION
We take advantage of the difference in vapor pressures of two components of a solution in the process of fractional distillation.
If we plot the boiling point of mixtures of benzene and toluene, we obtain a graph as shown in the lower curve of Figure 22-la. The boiling point of each mixture is, of course, the temperature at which the sum of the two vapor pressures equals 101 kPa.
At each of these points, however, the vapor phase would be richer in the more volatile component. Let us calculate the composition of the vapor in equilibrium with the liquid at each of these points, and plot the data on the same graph (upper curve of Figure 22-la).
Look at Figure 22-lb. Consider boiling a solution of benzene and toluene in which the mole fraction of benzene is 0.3. It will boil at the temperature represented by point A. The vapor in equilibrium with that solution will have the composition represented by point B which is at the same temperature. It is richer in benzene than the original solution because benzene is more volatile than toluene.
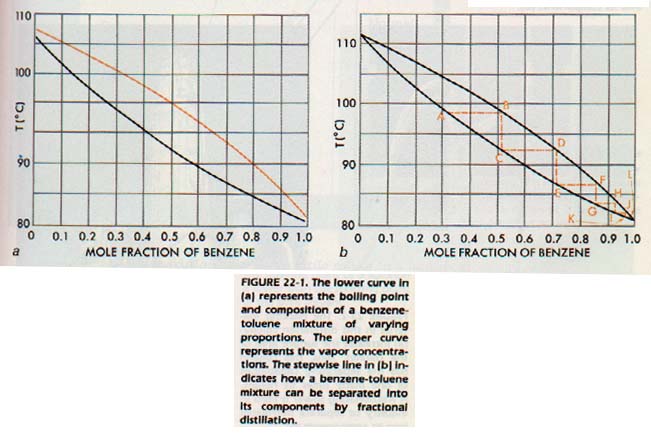
If we condense this vapor, we will obtain a solution which will boil at point C. It will produce a vapor composition D. This process can be continued until nearly benzene is obtained as vapor, and almost pure toluene behind.
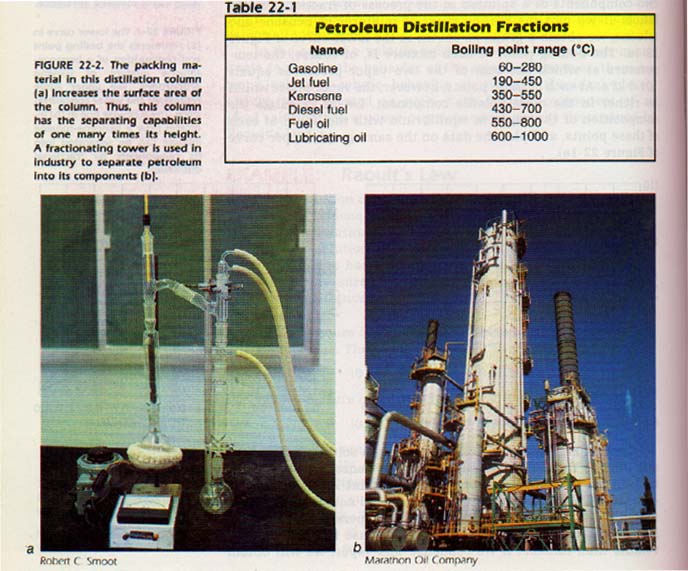
It is possible to construct a distillation apparatus which separate distillations for each step are not necessary. structure of the apparatus is such that each step takes place a separate section of the equipment. In the laboratory, a distillation apparatus is often used in separating volatile liquids. In industry, a fractionating tower is used for the same on a commercial scale. Petroleum is separated into useful products by fractional distillation.
22:3 BOILING POlNT AND FREEZlNG POlNT
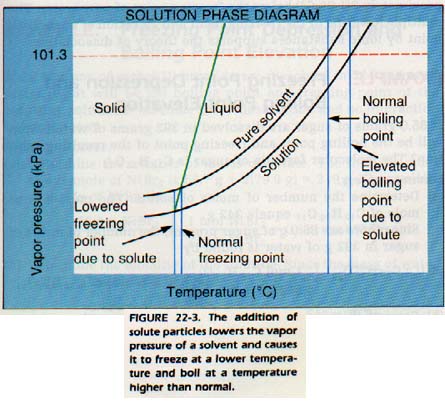
The presence of nonvolatile solute particles at the surface causes the boiling point of a solution to be raised. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid equals the atmospheric pressure.
In a solution, then, a higher temperature is needed to put enough solvent particles into the vapor phase to equal atmospheric pressure.
The boiling point of a solution is, therefore, higher than that of the pure solvent.
How does the addition of a nonvolatile solute affect the freezing point of a solution?
The freezing point is the temperature at which the vapor pressure of the solid and liquid are equal.
Since the addition of solute particles lowers the vapor pressure, the vapor pressures of the solid and liquid will be equal at a lower temperature. Solutions, then, will freeze at a lower temperature than the pure solvent alone.
In summary, the addition of a nonvolatile solute to a liquid causes both a boiling point elevation and a freezing point depression. Both boiling point elevation and freezing point depression occur because the vapor pressure of the solvent is lowered by the solute. These changes depend only on the concentration of the solute particles, and not upon the chemical composition of the solute.
We will now consider some general quantitative statements which can be made about these changes.
22:6 OSMOTlC PRESSURE
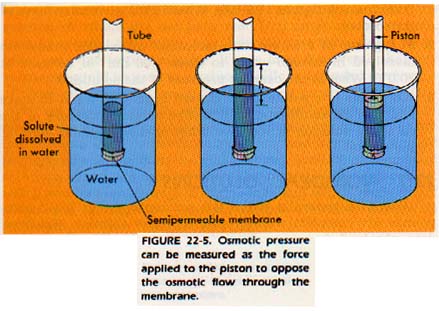
There is another colligative property which is of great importance in living systems. Consider Figure 22-5. Two liquids are separated by a thin film called a membrane. One liquid is a pure solvent and the other liquid is a solution (with the same solvent). The membrane separating the liquids is a special kind of membrane called a semipermeable membrane.
Semipermeable membranes will allow small particles (ions and molecules) to pass through, but will stop large molecules.
As a result of an unequal passing of particles, a pressure difference builds up between the two sides of the membrane. This pressure is called the osmotic pressure of the solution.
Osmotic processes are very important in the human body. Absorption of the products of digestion and the operation of the kidney as a waste remover are two osmotic processes vital to life.
22:8 COLLOlDS AND PHASES
In 1861, Thomas Graham, an English chemist, tested the passage of different substances through a parchment membrane. He found that one group of substances passed readily through the membrane, and another group did not pass through it at all.
He called the first group crystalloids and the second group colloids.
The name colloid means glue like, and glue was one of the substances which did not pass readily through the membrane.
Graham thought the ability or inability to pass through the membrane was due to particle size. It was later discovered that any substance could be used to produce a colloid. Included were some of those substances Graham had classified as crystalloids.
Colloids are now defined as mixtures composed of two phases of matter, the dispersed phase and the continuous phase. They are intermediate class between suspensions and solutions.

Colloid particles are larger than the single atoms, ions, or molecules of solutions. They are smaller than the particles of suspensions, which can be seen through a microscope and which settle out of suspension on standing. Colloids include materials labeled as emulsions, aerosols, foams, and gels.

22:9 COLLOlDAL SlZE
Colloidal particles are too small to be seen with a microscope. In 1912, Richard Zsigmondy, a German professor of chemistry, designed the ultramicroscope. Using the ultramicroscope, it is possible to "see" colloidal particles. If a finely ground substance is placed in water, one of three things will happen.
First, it may form a true solution which is simply a dispersion of atoms, molecules, or ions of the substance into a solvent. The size limit of the particles in a true solution is about 1 nm.
Second, the particles may remain larger than 100 nm. These particles are large enough to be seen with a microscope. They are strongly affected by gravity and gradually fall to the bottom of the container. Since the particles are temporarily suspended and settle out upon standing, this mixture is called a suspension.
Particles from 1 to 100 nm in size usually remain dispersed throughout the medium. Such a mixture is called a colloid. Colloids are neither homogeneous nor heterogeneous. They represent a transition between homogeneous solutions and heterogeneous suspensions. However, they are considered heterogeneous (with the medium as one phase and the dispersed substance as a separate phase).

Actually, it is not enough to refer to colloids as being composed of particles in the above size range. Substances show unusual properties even when only one of the three dimensions of the particles is in the colloidal range. Included are thin sheets as well as minute particles. Colloid chemistry is defined as the study of the properties of matter whose particles are colloidal in size in at least one dimension.

22:10 PROPERTlES OF COLLOlDS
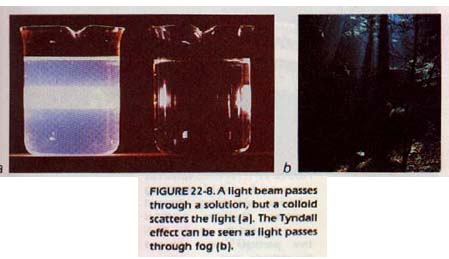
If a beam of light is allowed to pass through a true solution, some of the light will be absorbed, and some will be transmitted. The particles in solution are not large enough to scatter the light.
However, if light is passed through a colloid, the light is scattered by the larger, colloidal particles. The beam becomes visible from the side. This effect, called the Tyndall effect.
You may be familiar with this effect in seeing the beam of a searchlight in the night air (suspended water droplets in air). You may also have observed it as the sunbeam coming through a hole in the blinds (suspended dust particles in the air).
Colloids have another interesting property. If you could look through an ultramicroscope, you would notice that the colloidal particles are in continuous motion.
This motion, called Brownian motion, is a random motion of the particles. This motion is caused by their constant bombardment by the smaller molecules of the medium. The motion is the result of the collision of many molecules with the particle.
It is as if a large crust of bread is moved back and forth as first one fish and then another hits the bread from opposite sides. Brownian motion is named in honor of Robert Brown, a biologist. Brown first noticed it while observing the motion of particles in a suspension of pollen grains in water.
As noted in Chapter 17, particles on the surface of a liquid are subject to unbalanced forces. Atoms, ions, or molecules at the surface of solids are also subject to unbalanced forces.
As a result, solid and liquid surfaces tend to attract and hold substances with which they come into contact. This phenomenon is called adsorption.
The stationary phase in chromatography operates through adsorption. Colloidal particles, because of their small size, have an extremely large ratio of surface to mass.
A cube with a volume of 1 cm3 has a surface area of 6 cm2. If that cube is cut into 1OOO smaller cubes of equal size, the surface area of that same amount of matter is now 60 cm2.
If we subdivide these cubes until they measure 10 nm on edge, the surface area has risen to 6 000 000 000 cm2. Such a large surface area makes colloidal particles excellent adsorbing materials, or adsorbents. Dispersed particles have the property of adsorbing charge on the surface.
If a colloid is subjected to an electric field, a migration of the particles can be observed. The positive particles are attracted to the cathode. The negative particles are attracted to the anode. This migration, called electrophoresis, is evidence that colloid particles are charged.
The separation of amino acids and peptides obtained in protein analysis is accomplished rapidly using electrophoresis. The process is also common in nucleic acid research.
SUMMARY
1. The vapor pressure of a solution is the sum of the vapor pressures of its components. The vapor pressure of a component of a solution is its normal vapor pressure multiplied by its mole fraction in the solution.
2. Many substances can be separated by taking advantage of the difference in their vapor pressures. The process used is called fractional distillation.
3. Solutes affect the vapor pressure, boiling point, freezing point, and osmotic pressure of a solvent.
4. Colloid particles range between 1 and 100 nm in at least one dimension.
5. A colloid is composed of two phases: the dispersed phase and the continuous phase.
6. Colloids possess some unusual properties. They can scatter light (Tyndall effect), undergo constant random motion (Brownian motion), and act as excellent adsorbing materials.
More on Colligative Properties:
For a PowerPoint presentation Click Here.
............... Why not drink Distilled Water?
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page