Quantitative Relationships
![]()
Quantitative Relationships
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
QUANTITATIVE RELATlONSHlPS
Chemists are constantly faced with the question, "How much?" How much of an element is present in a compound? How much water is contained in a hydrate? How much of each element or compound is required to prepare a new compound?
The mass relationships between reactants and products in chemical changes are an important concern to chemists. Several questions arise here. How much of one reactant is needed to combine with a given amount of another reactant? How much product is produced from a specific amount of reactant? There are other, similar, questions. Fortunately, all these questions can be answered using the same procedure.
7:1 MASS-MASS RELATIONSHIPS
A chemical equation is simply a shorthand expression which gives information about a reaction. Once a balanced equation is written, it can be used to help solve problems involving the reaction. The coefficients of a balanced equation give the relative amounts (in moles) of reactants and products. Calculations to find the masses of materials involved in reactions are called mass-mass problems.
EXAMPLE: Mass-Mass
How many grams of silver chloride can be produced from reaction of 17.0 g of silver nitrate with excess sodium solution?
Solving process:
Step 1. We are given silver nitrate and requested to find chloride. What do we know which
We then write a balanced equation to represent reaction which occurs. Silver nitrate is reacting with sodium chloride. Since we have compound plus compound, we that a double displacement reaction will occur.
AgN03 + NaCl ---> AgCl + NaNO3
Step 2. In Chapter 6, we thought of equations as written in of individual atoms and formula units. However, we can consider the coefficients in the equation as indicating the number of moles or formula units which take part in the reaction.
The equation above indicates that one formula unit of silver nitrate will produce one formula unit of silver chloride. It also indicates that one mole of silver nitrate formula units will produce one mole of silver chloride formula units.
The problem states that an excess of sodium chloride is used. This statement shows us that all the silver nitrate will react.
Let us review where we are. We are given grams of silver nitrate. We are asked to find grams of silver chloride. Silver nitrate and silver chloride are related by the equation. The equation is read in units of moles.
Step 3. Our solution begins by converting the grams of silver nitrate to moles. We use the Atomic Masses Table to determine the formula mass of AgNO3 which is 170 g.
Step 4. We have now converted the silver nitrate to units that will enable us to relate it to the silver chloride. We use the equation to find the mole ratio to use in going from silver nitrate to silver chloride. The equation tells us that one mole of silver nitrate will produce one mole of silver chloride. Using this fact gives us a partial solution.
Step 5. We have now arrived at silver chloride which is the substance we were asked to find. However, we were to find the answer in grams. To complete the problem, we must convert the moles of silver chloride to grams. So we must multiply the number of moles of silver chloride by the molecular mass (formula mass) of silver chloride (the grams per mole of silver chloride).
This problem involves finding the mass of one substance from a given mass of another substance. Problems of this type are called mass-mass problems. All mass-mass problems can be solved in this way. This example and other quantitative studies of chemical reactions are called stoichiometry (stoh kee AHM Uh tree). Let us review the process.
Step 1. Write the balanced equation.
The first step in the solution of any problem is to write a balanced equation for the correct reaction. In this section, we will always assume that only one reaction occurs and that all of one reactant is used. In practice, several reactions may occur, the actual reaction may not be known, or all the reactant may not react.
Step 2. Find the number of moles of the given substance. Express the mass of the given substance in moles by dividing the mass of the given substance by its formula mass.
Step 3. Inspect the balanced equation to determine the ratio of moles of required substance to moles of given substance. For example, look at the following equation.
2H2 + 02 ---> 2H2O
In this reaction, 1 mole of oxygen reacts with 2 moles of hydrogen. From the same equation, 1 mole of oxygen will produce 2 moles of water. Also, 2 moles of water are produced by 2 moles of hydrogen. Once the equation is balanced, only the reactants and products directly involved in the problem should be in your calculations. Multiply the moles of given substance by the ratio.
Step 4. Express the moles of required substance in terms of grams.
7:2 ENERGY AND CHEMlCAL CHANGE
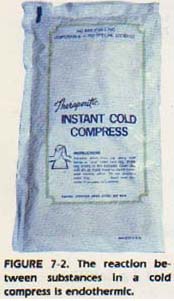
Chemical changes are always accompanied by a change in energy. If heat energy is absorbed in a reaction, the reaction is endothermic. The products, therefore, are higher in energy than the reactants. On the other hand, if heat energy is given off by a reaction, the reaction is exothermic.
In this case, the products are lower in energy than the reactants. For example, the calcium hydroxide-phosphoric acid reaction is exothermic. If a thermometer is placed in the reaction vessel, the temperature will rise as the reaction occurs. Heat energy is given off. The products, calcium phosphate and water, are at a lower energy state than the reactants.
Quantitative measurements of energy changes are expressed in joules or calories. The joule (J) is an SI unit. It is a derived unit rather than a base unit.
Both endothermic and exothermic reactions require a certain minimum amount of energy to get started. This minimum amount of energy is called the activation energy. Without it, the reactant atoms or molecules will not unite to form the product and the reaction does not occur.
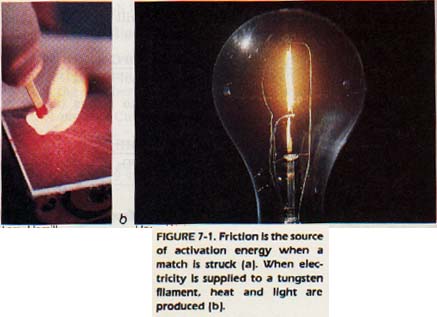
Chemical reactions have many possible sources for their activation energy. When a match is struck, friction produces enough heat to activate the reactants on the match head. As a result the match ignites. In photography, light is the source of the activation energy.
7:3 HEAT MEASUREMENT
Experimentally, the energy changes of chemical reactions are measured in a calorimeter, Figure 7-4.
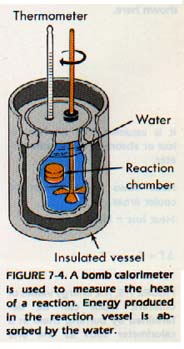
The energy quantities involved in various physical changes can also be measured in a calorimeter. To change the temperature of a substance, heat must be added or removed.
Some substances require little heat to cause a change in their temperature. Other substances require a great deal of heat to cause the same temperature change. For example, one gram of water requires 4.18 joules of heat to cause a temperature change of one Celsius degree. It takes only 0.903 joule to raise the temperature of one gram of aluminum one Celsius degree.
The amount of heat needed to raise the temperature of 1 g of a substance by 1Co is called the specific heat capacity (Cp) of the substance.
Every substance has its own specific heat capacity. The amount of heat required to raise the temperature of one gram of water one Celsius degree is 4.18 joules. The specific heat capacity of water is 4.18 J/g • Co.
Specific heat capacities are given in joules per gram-Celsius degree (J/g • Co. Tables A-3 and A-5 of the Appendix list the specific heat capacity of some substances. The specific heat capacity can be used in calculations involving the change in temperature of a specific amount of substance.
Another heat quantity, often used by chemists is the heat capacity. This is quantity is the heat needed to raise temperature of 1 mole of a substance by 1Co. In the case monatomic element, the term atomic heat capacity is used of molar heat capacity. The molar (or atomic) heat capacity of substance is found by multiplying the specific heat capacity the substance by its formula (or atomic) mass. All of the changes we have discussed are measured in calorimeters.
There are several kinds of calorimeters. A calorimeter containing water is often used to measure the heat absorbed or released by a chemical reaction. Heat energy from a chemical reaction in the cup in the calorimeter causes a change in temperature of the water in the calorimeter. The temperature change of the water is used to measure the amount of heat absorbed or released by the reaction.
The product of the specific heat capacity of the water, the temperature change of the water, and the mass of the water gives the change in heat energy. The heat energy released or absorbed by water is calculated using the following equation:
Δis delta. It means "a change in".
Q = mcΔt
Where Q is the heat in joules or calories, m is the mass in grams, c is the specific heat from the table, and Δt is the temperature change in Co.
For example, to heat 40.0 g of water from 20.OoC to 36.OoC requires: 2680 J.
Click here for Table A-5 Specific Heats of Substances
7:5 HEAT OF CHEMlCAL REACTlON
Balanced chemical equations can be used to predict the relative amounts of reactants and products in a reaction. A balanced chemical equation can also be used to calculate the energy absorbed or released during a chemical reaction.
When carbon (in the form of coal) is burned, heat is released.
C + O2 ---> CO2 + heat (393.5 kJ)
One mole of carbon reacts with one mole of oxygen to produce one mole of carbon dioxide and 393.5 kJ of heat. The heat released (393.5 kJ) is called the heat of reaction.
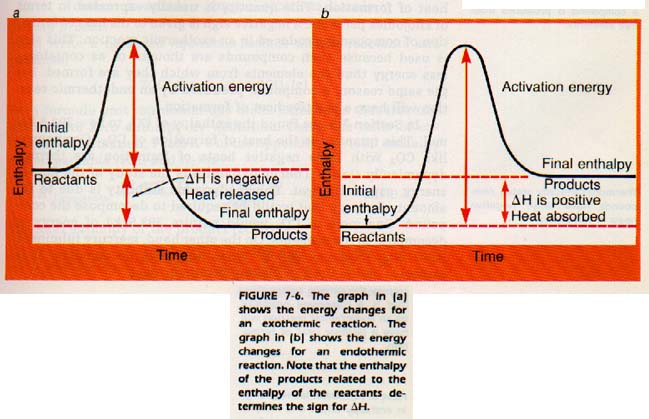
Since heat is released during this reaction, the product (CO2) must contain less energy than the reactants (C and 02). we think of the heat as coming from the reactants, we can assign each reactant a heat content. The heat content or represented by the symbol H.
We cannot measure enthalpy (heat content) in absolute We can only measure changes in enthalpy. The height of a hill cannot be measured in absolute terms either. We can describe the level of the surrounding plain or we say it is a certain number of meters above sea level.
The figure we give depends upon what standard of reference is used. In measuring heat content, we use the heat content of a free element at atmospheric pressure and 25oC as our standard.
We arbitrarily define this heat content as zero. If carbon is burned in oxygen, chemical potential energy is converted into heat. We assign zero heat content to the elements carbon and oxygen. Therefore, the molar heat content of carbon dioxide must be -393.5 kJ/mol. Thus, carbon dioxide has a heat content or enthalpy of -393.5 kJ/mol.
Different substances have differing enthalpies because their atoms are joined in different ways. When a reaction takes place, the atoms are rearranged. The new arrangement may represent more or less enthalpy than the original arrangement. Thus, energy must be given off or absorbed.
7:6 HEAT OF FORMATION
The preceding reaction involves the formation of a compound from its elements. The change in enthalpy, ΔH, when one mole of a compound is produced from the free elements is known as the heat of formation.
This quantity is usually expressed in terms of kilojoules per mole. A negative sign is given to the heat of formation of compounds produced in an exothermic reaction. This sign is used because such compounds are thought of as containing less energy than the elements from which they are formed.
For the same reason, a compound produced by an endothermic reaction will have a positive heat of formation.
In Section 7:5, we found the enthalpy of CO2 to be -393.5 kJ/mol. This quantity is the heat of formation of CO2.
Compounds like CO2 with large negative heats of formation are thermodynamically stable.
Thermodynamics is the study of the flow of energy, especially heat. Thermodynamic stability is due to the amount of energy that would be required to decompose the compound.
One mole of CO2 would require 393.5 kJ of energy to decompose to the elements. On the other hand, mercury fulminate, Hg(OCN)2, produces 268 kJ when one mole decomposes. It is explosive and is used in making detonator caps.
SUMMARY
1. The balanced equation indicates the ratio of moles of reactants to moles of products in the reaction and is used as the basis for solving mass problems.
A chemical change is always accompanied by an energy change. Energy is absorbed in an endothermic reaction and given off in exothermic reaction.
3. Activation energy is the energy required to start a chemical reaction.
4. The specific heat capacity of a substance is the heat required to raise the temperature of 1 g of the substance 1Co
5. The heat energy transferred when a quantity of matter changes temperature is Q = mcΔt. This value is expressed using an energy called joules of calories.
6. The specific heat capacity of water is 4.18 J/g • Co.
7. The change in enthalpy of a system is the energy absorbed or produced during a chemical reaction. This change is represented by ΔH.
8. The heat of formation ΔH of a compound is the heat released or absorbed when 1 mole of the compound is formed from its elements
9. A thermodynamically stable compound has a large negative heat formation.
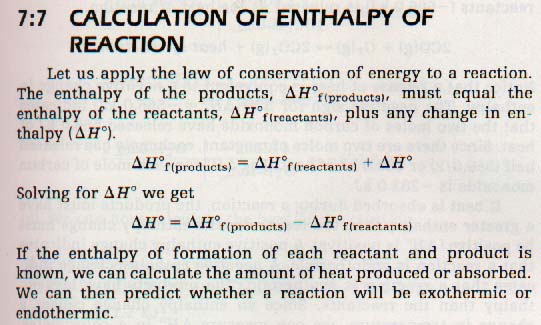
10. The enthalpy change, (ΔH) for a reaction is the difference between enthalpy of the products and the enthalpy of the reactants.
More on Quantitative Relationships:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & PowerPoints ***
Chemistry *** Class Notes & PowerPoints ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
![]() Return to the Big Chem Page
Return to the Big Chem Page