Chemical Bonding
![]()
Chemical Bonding
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
12:1 CHEMlCAL BONDlNG
In Chapter 10, we looked at the ways atoms are classified. We found that some atoms tend to give up electrons and become positive ions, while other atoms tend to gain electrons and become negative ions. We also discovered that some atoms tend to share electrons. In this chapter, we want to look at these processes in detail.
12:1 DETERMINING lONlZATlON ENERGY
Our model of the atom was developed partly from determining the energy needed to remove the most loosely held electron from an atom. The experiments involve shooting electrons through a vapor. A substance is vaporized in a space between two electrodes as shown in Figure 12-1.
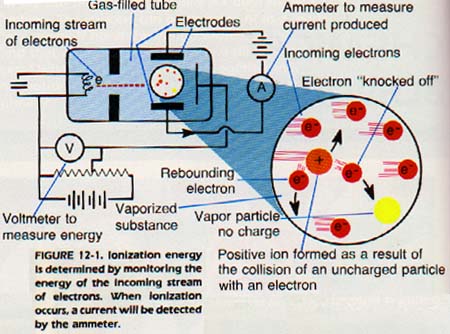
No current flows through the vapor between the electrodes because the vapor particles have no charge. When this vapor is bombarded with a stream of electrons, some of the bombarding electrons will collide with atoms. The collision will result in "knocking off" electrons from atoms.
This process produces positively charged ions. The positive ions will move toward the negative electrode, and a current will be detected. This current indicates that the vapor has been ionized.
In order to determine the necessary energy for ionization, the energy of the electron stream is increased slowly. When a current is detected, the energy of the electrons which first caused the ionization is noted. That value is the energy necessary to remove completely the most loosely held electron from an atom of the element being bombarded. This energy is called the first ionization energy of that element. It is measured in kilojoules per mole (kJ/mol).
12:2 FlRST lONlZATlON ENERGlES
The first ionization energies of the first ninety-five elements are graphed in Figure 12-2. Note that the ionization energies, like many other properties of the elements, are periodic. In fact, the relative ionization energies of two elements can be predicted by referring to their positions in the periodic table.
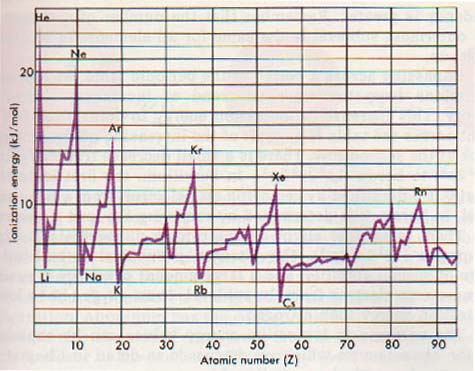
The ionization energy tends to increase as atomic number increases in any horizontal row or period. In any column or group, there is a gradual decrease in ionization energy as atomic number increases. Note, for example, the gradual decrease in ionization energy in the alkali metal series, lithium through cesium. The same trend is seen in the noble gas series, helium through radon.
A metal is characterized by a low ionization energy. Metals are located at the left side of the table.
An element with a high ionization energy is a nonmetal. They are found at the right side of the table.
These ionization energies provide strong evidence for the existence of energy levels in the atom. Our theories of structure are based on experimental results such as ionization energies and atomic spectra. The experimental evidence came first, then the model of structure.
Look at Table 12-1. Notice that the ionization energies decrease as you go down a column of the periodic table (for instance, lithium, sodium, potassium). The increased distance of the outer electrons from the nucleus and the shielding effect of the inner electrons tend to lower the ionization energy.
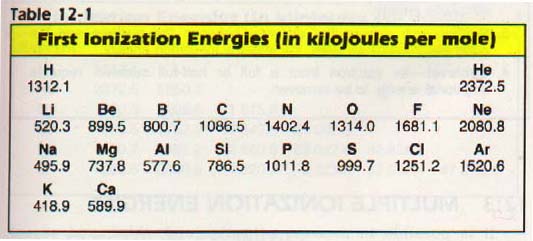
Though it appears that the increased nuclear charge of an element with a greater atomic number tends to increase ionization energy, the lowering tendency is greater.
Remember that the number of electrons in the outermost sublevel is the same for all elements in a column or group.
In passing across a period of the periodic table, we see some deviations from the expected trend of increasing ionization energy. This increase in ionization energy in going from left to right across the table is a result of the increasing nuclear charge.
Look at the second row. There is a small decrease from beryllium (ls22s2) to boron (ls22s22p1).
In beryllium, the first ionization energy is determined by removing an s electron from a full s sublevel. In boron, it is determined by removing the lone p electron. There is another slight decrease from nitrogen (ls22s22p3) to oxygen (ls22s22p4).
The nitrogen p sublevel is half-full (a state of special stability) and a large amount of energy is needed to remove an electron from the sublevel. Thus, oxygen has a lower ionization energy than nitrogen.
The patterns in ionization energy values can be explained by the same factors which we discussed in detail in Chapter 10 in connection with the periodic table.
12:3 MULTlPLE lONlZATlON ENERGIES
It is possible to measure other (second, third, and so on) ionization energies of an atom. These measurements give us the same evidence for atomic structure as first ionization energies.

For example, the second ionization energy of aluminum is about three times as large as the first, as shown in Table 12-3. The difference can be explained by the fact that the first ionization removes a p electron and the second removes an s electron from a full s sublevel.
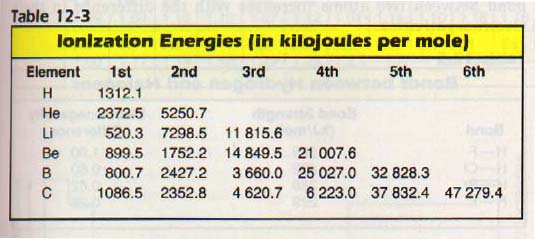
The third ionization energy is about one and two-thirds times as large as the second. The second and third electrons are in the same sublevel. Yet, the third electron's ionization energy is greater because the nuclear charge remains constant as we remove electrons.
As a result, the remaining electrons are more tightly held. The fourth ionization energy is about four times as large as the third. Why is the jump so large between the third and fourth ionization energies?
Let us look at the electron configuration. Aluminum has the configuration ls22s2 2p6 3s2 3p1.
The fourth electron would come from the full second energy level which is closer to the nucleus. The 2s22p6 level with eight electrons is stable. Thus a large amount of energy will be required to remove that fourth electron.
We have looked only at the aluminum atom, but the same reasoning can be used to explain similar data for other elements. This information can be applied as evidence for our theories of atomic structure.
12:4 ELECTRON AFFINITIES
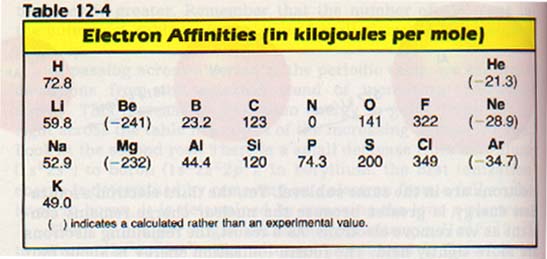
Now consider an atom's attraction for additional electrons. The attraction of an atom for an electron is called electron affinity. The same factors which affect ionization energy will also affect electron affinity.
In general, as electron affinity increases, an increase in ionization energy can be expected.
Metals have low electron affinities. Nonmetals have the high electron affinities as shown in Table 12-4.
12:5 ELECTRONEGATlVlTY
Both electron affinity and ionization energy deal with isolated atoms. Chemists need a comparative scale relating the abilities of elements to attract electrons when their atoms are combined.
The relative tendency of an atom to attract electrons to itself when bound with another atom is called its electronegativity. Elements are assigned electronegativities on the basis of experimental tests.
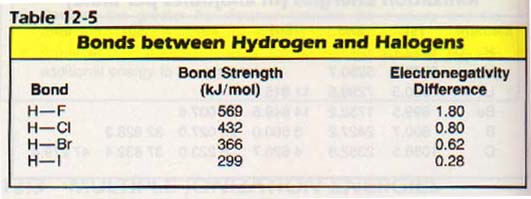
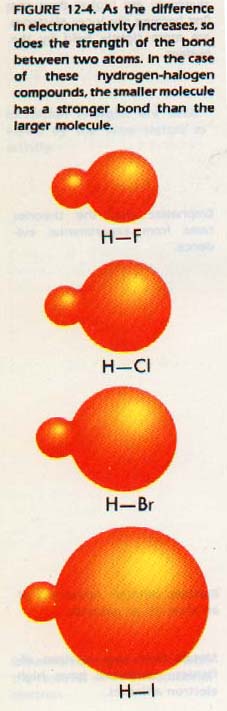
Many chemical properties of the elements can be organized in terms of electronegativities. For example, the strength of bond between two atoms increases with the difference in electronegativities.
Electronegativities of elements are influenced by the same factors affecting ionization energies and electron affinities.
It is possible to construct an electronegativity scale using first ionization energies and electron affinities of the elements. Examine Table 12-6 which represents average values from several calculations. It shows that the variation in electronegativity follows the same trends as the ionization energies and the electron affinities. The most active metals (lower left) have the lowest electronegativities.
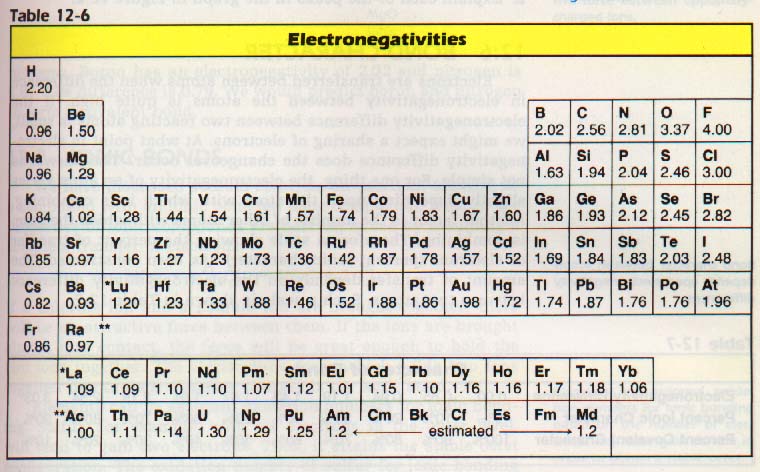
Fluorine has the highest electronegativity of all the elements. Consider a reaction between two elements. Their relative attraction for electrons determines how they react. We can use the electronegativity scale to determine this attraction. Since electronegativity represents a comparison of the same property for each element, it is a dimensionless number.
12:6 BOND CHARACTER
Electrons are transferred between atoms when the difference in electronegativity between the atoms is quite high. If the electronegativity difference between two reacting atoms is small, we might expect a sharing of electrons.
At what point in electronegativity difference does the changeover occur? The answer is not simple. For one thing, the electronegativity of an atom varies slightly depending upon the atom with which it is combining. Another factor is the number of other atoms with which the atom is combining. Therefore a scale showing the percent of transfer of electrons (percent ionic character) has been constructed. The amount of transfer depends on the electronegativity difference between two atoms.
When two atoms combine by transfer of electrons, ions are produced. The opposite charges of the ions hold them together.
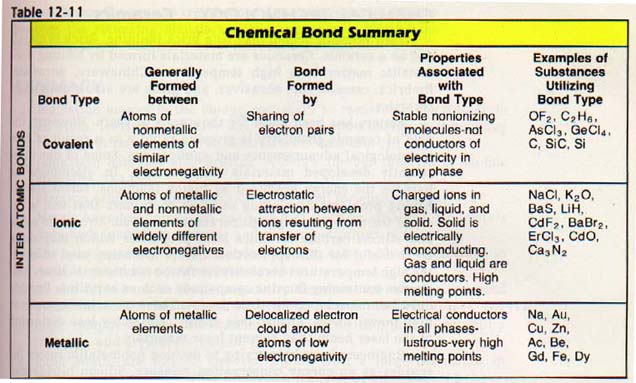
When two elements combine by electron transfer, they are said to form an ionic bond.
If two elements combine by sharing electrons, they are said to form a covalent bond.
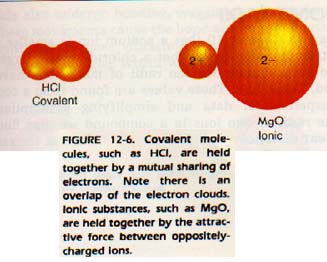
12:7 lONlC BONDS
We have discussed sodium chloride, an excellent example of a compound with an ionic bond (an ionic compound). Most of the properties of ionic compounds are best explained by assuming a complete transfer of electrons.
2Na+ + Cl2 ---> 2Na+ + 2Cl-
If a chloride ion and a sodium ion are brought together, there will be an attractive force between them. If the ions are brought almost into contact, the force will be great enough to hold the two ions together.
The electrostatic force which holds two ions together due to their differing charges is the ionic bond.
Elements can be assigned oxidation numbers for ionic bonding. Sulfur, for example, with six electrons in the outer level, will tend to gain two electrons. Thus, it attains the stable octet configuration. The oxidation number of sulfur for ionic bonding is 2-. The negative two is its electric charge after gaining two electrons.
Ionic compounds are characterized by high melting points and the ability to conduct electricity in the molten state. They tend to be soluble in water and usually crystallize as sharply defined particles.
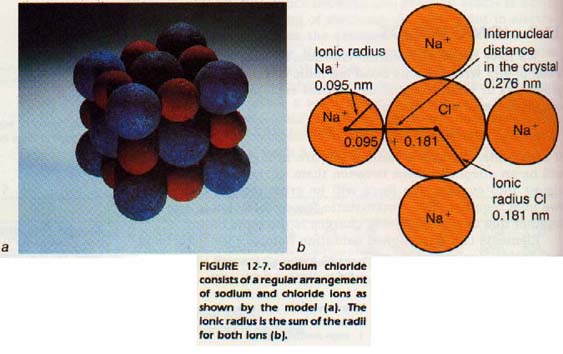
12:8 lONlC RADll
We saw in Chapter 10 that a sodium ion is smaller than a sodium atom. We also found that a chloride ion is larger than a chlorine atom. Values for the radii of many ions have been determined, Table 10-9.
These values are found from a combination of experimental data and simplifying assumptions. By adding the radii of two ions in a compound we may find their internuclear distance in a crystal. Remember that the radii are not fixed values. One reason for their variability is the "fuzziness" of the electron cloud. Another reason is the effect each ion has an its neighbor ions.
12:9 COVALENT BONDS
Atoms with the same or nearly the same electronegativities tend to react by sharing electrons. The shared pair or pairs of electrons constitute a covalent bond. Covalent compounds typically have low melting points, do not conduct electricity, and are brittle.
When two or more atoms bond covalently, the resulting particle is called a molecule.
The line joining the nuclei of two bonded atoms in a molecule is called the bond axis as shown in Figure 12-13.
If one atom is bonded to each of two other atoms, the angle between the two bond axes is called the bond angle. The distance between nuclei along the bond axis is called the bond length. This length is not really fixed, because the bond acts much as if it were a stiff spring. The atoms vibrate as though the bond were alternately stretching and shrinking as shown
in Figure 12-8.
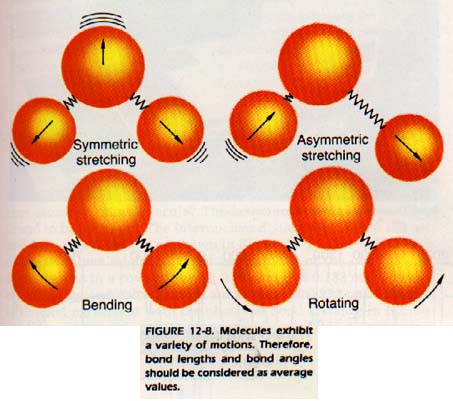
Bonds also undergo bending, wagging, and rotational vibrations. These movements cause the bond angles and length to vary. The amplitudes of these vibrations are not large and the bond lengths and bond angles which we measure are average values.
We may think of them as the values for a molecule completely at rest. However, molecular motion never entirely ceases.
We have learned much of what we know about the structure af molecules from infrared spectroscopy. Recall from Figure 8-14 that infrared wavelengths lie in a region of the electromagnetic spectrum between radio waves and visible light waves.
The wavelengths vary from 700 nm to over 50 000 nm. A molecular compound can be identified by the infrared radiation it absorbs or transmits. Each molecular compound has its own infrared spectrum which is different from that of any other compound. The infrared (IR) spectrum indicates energy changes in the bonding between the particles af the molecule.
At specific frequencies the atoms of the molecule stretch, twist, wag, and bend around the bonds joining them. Radiation of these wavelengths corresponding to those frequencies will be absorbed. The energy absorbed must agree in frequency with the natural frequency of vibration of the molecule.
In using the IR spectrophotometer, a sample of the compound is subjected to varied wavelengths of IR radiation. The various wavelengths absorbed by the compound are measured and recorded graphically. A unique continuous absorption spectrum can be plotted for each molecular compound. Comparison with spectra will reveal the identity of the compound, just as fingerprints reveal the identity of a person.
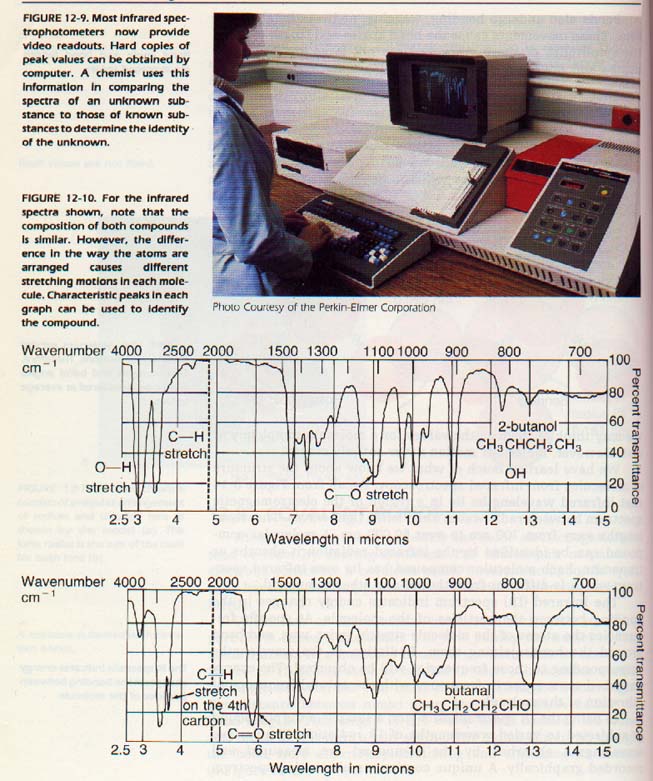
2:10 COVALENT RADll
It is possible, by experiment, to determine the internuclear distances between two bonded atoms. For example, consider iodine(VII) chloride, ICI. What are the radii of the iodine and chlorine atoms in this molecule?
The internuclear distance in ICI is found to be 0.230 nm. The internuclear distance in Cl2 is 0.198 nm, and in I2 it is 0.266 nm as shown in Figure 12-11. One half of each of these values might be taken as the radii of the chlorine and iodine atoms in a covalent bond: 0.099 nm and 0.133 nm.
The sum of the iodine and chlorine radii would then be 0.232 nm. This sum is in good agreement with the observed bond distance in ICI. Covalent radii are only approximates; the value for hydrogen is less reliable than that for other atoms.
Nevertheless, these radii are very useful in predicting bond lengths in molecules. Table 12-8 gives the covalent radii for some common atoms. Table 12-9 gives the bond lengths for some molecules. See for yourself how well the predicted bond lengths agree with the measured ones.

Remember that covalent radii are used to find the internuclear distance between atoms bonded to each other. Like electronegaltivities covalent radii are average values. The radius of a particular atom is not constant. Its size is influenced by the other atom or atoms to which it is bonded.
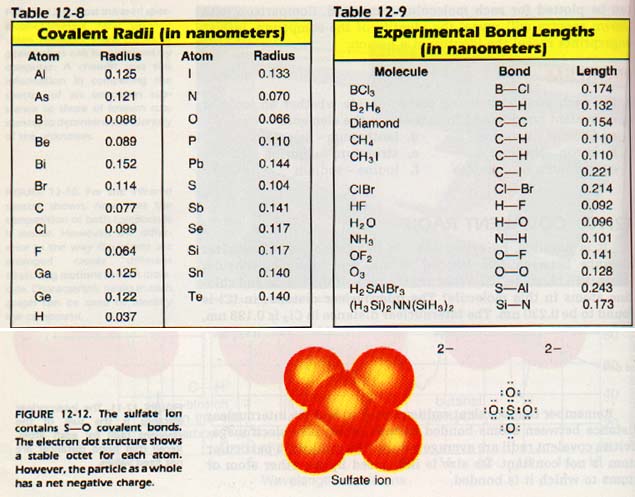
12:11 POLYATOMlC lONS
There are a large number of ionic compounds made of more than two elements. In these compounds, one ion consists of two or more atoms covalently bonded. However, the particle as a whole possesses an overall charge.
For example, consider the hydroxide ion, OH-1. The oxygen atom is bonded covalently to the hydrogen atom. The hydrogen atom is stable with two electrons in its outer level.
The hydrogen atom contributes only one electron to the octet of oxygen. The other electron required for oxygen to have a stable octet is the one which gives the 1- charge to the ion. Although the two atoms are bonded covalently, the combination still possesses charge. Such a group is called a polyatomic ion. Polyatomic ions form
Ionic bonds just as other ions do. Table 4-4 gives some of the more common polyatomic ions with their charges.
12:12 VAN DER WAALS RADll
A certain minimum distance must be maintained between atoms that are not bonded to each other. This limitation exists because the electron cloud of one atom repels the electron cloud of other atoms.
In effect, colliding free atoms and molecules act as if they had a rigid outer shell. This shell limits the closeness with which they may approach other atoms or molecules.
Since the covalent bond consists of shared electrons, bonded atoms can come closer than atoms which are not bonded as shown in Figure 12-13. The radius of this imaginary rigid shell of an atom is called the van der Waals radius. It is named for the Dutch 'physicist Johannes van der Waals.
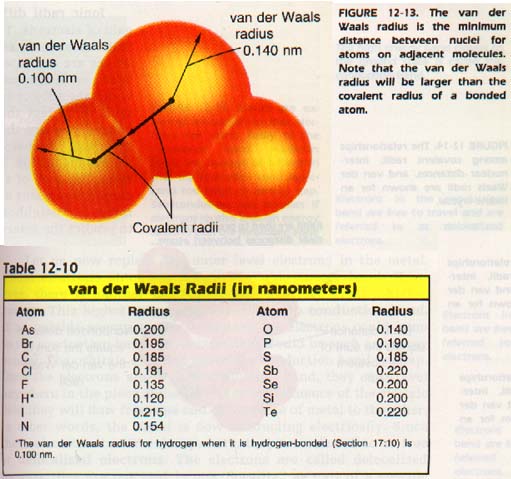
1213 SUMMARY OF RADll
Thus far, we have studied four radii-- atomic, ionic, covalent, and van der Waals. How do these various measurements differ? How are they the same?
Atomic radii are measured in one of two ways. First, they may be measured on individual atoms in the gaseous state. On the other hand, they may be measured on atoms in metallic crystals. These atoms have special characteristics which will be discussed in Section 12:14.
12:14 SPECIAL PROPERTlES OF METALS
The properties of metals are not explained by any of the bonding properties already considered. One of these properties is the ability to conduct electricity quite readily. Good electric conductivity indicates a ready source of electrons in metals. We can use the following model to describe a piece of metal. Imagine metal atoms without their outer level electrons.
These positive ions are packed together and their empty outer-level orbitals interact with each other. Each outer level is split into closely spaced energy levels. These splits are so small that we actually have bands of possible energies. These energy bands are separated from each other by small energy gaps called forbidden zones as shown in Figure 12-15.
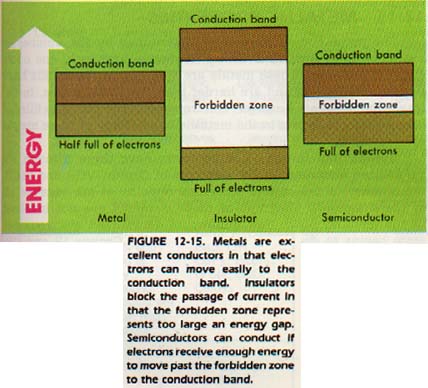
Let us now replace the outer level electrons in the metal. Electrons enter the lowest available energy band. However is another band representing a just slightly higher .
This higher energy band is called the conduction band.
When outside energy source is applied, the electrons can jump the conduction band. An electric field would be such an energy. The orbitals of the atoms in the conduction band overlap.
Once the electrons are in this conduction band, they can travel. in the piece of metal. Under the influence of the electric they will flow from one end of the piece of metal to the other. In other words, the metal is now conducting electrically.
Since electrons can travel anywhere, they are referred to as free delocalized electrons.
The electrons are called delocalized because they are not held in one "locality" as part of a specific or bond.
Geese on the leeese.
The delocalized electrons act as a negative cloud and holding the positive lons. It is this negative which constitutes the metallic bond.
In nonmetals, the forbidden zone represents a large quantity energy. As a result, nonmetals do not conduct electricity. They called insulators since they block the passage of an electric
There is another class of elements, called semiconductors. these elements, all the outer electrons form covalent bonds. the forbidden zone in these elements is small. It is
12:15 METALLlC PROPERTIES
The properties of metals are determined by the number outer electrons available. Group IA metals have only one electron per atom. These metals are soft. Group IIA metals two outer electrons and are harder than Group IA metals. In the transition elements however, electrons from the partially filled d orbitals may take part in the metallic bond. Many of these metals are very hard.
Groups IIIB through VIB elements have three through six delocalized electrons. In the elements of Groups VIIB and VIILB, the number of delocalized electrons remains at six because all of the d sublevel electrons of these elements are not involved in the metallic bond.
The number of delocalized electrons per atom begins to decrease with the metals of Groups IB and IIB. Going from Groups IIIA through VIIIA, the nonmetals, the metallic properties decrease rapidly.
The strong metallic bond of our structural metals, such as iron, chromium, and nickel, makes them hard and strong.
In general, the transition elements are the hardest and strongest elements. It is possible to strengthen some of the elements with fewer delocalized electrons by combining them with other metals to form alloys. These alloys have properties different from those of pure elements.
SUMMARY
1. Ionization energy is the energy necessary to remove an electron from an atom, leaving a positive ion. First ionization energy is the energy required to remove the first electron from an atom.
2. Ionization energies also provide evidence for our theories of atomic structure.
3. Electron affinity is the attraction of an atom for electrons.
4. Metals have low ionization energies and electron affinities. Nonmetals have high ionization energies and electron affinities.
5. The relative tendency of a bonded atom to attract shared electrons to itself is called its electronegativity.
8. As the difference in electronegativity between two covalently bound atoms increases, the strength of the bond between them increases. Ionic compounds are characterized by high melting points, solubility in water, and crystal formation.
8. Ionic bonds are formed between atoms with a large difference in electronegativity. This bond involves a transfer of electrons.
9. The ionic radius is the best estimate chemists can make of the effective size of an ion.
10. Covalent bonds are formed between atoms with slight differences in electronegativity. This bond involves sharing of electrons.
11. The bond axis is a line joining the nuclei of two bonded atoms. The length of the bond axis is called the bond length. The angle between two bond axes is called the bond angle.
12. The infrared (IR) spectrophotometer can be used to determine molecular structure. Radiation with wavelengths 700 to 50 000 nm is absorbed in characteristic patterns by molecular bonds.
13. Covalent radii can be used to predict the distance between bonded atoms.
14. Polyatomic ions possess an overall charge just as other ions. However, they are composed of groups of atoms bonded covalently.
15. Compounds containing polyatomic ions are bonded ionically.
16. Electron clouds act as hard spheres when two nonbonded atoms approach each other. The radius of this imaginary sphere is called the van der Waals radius of the atom.
17. A metallic bond is formed between atoms with few electrons in the outer level. These electrons circulate as delocalized electrons and allow metals to carry an electric current.
18. The high number of delocalized electrons of such metals as iron, chromium, and nickel makes these metals very hard and strong. In the transition elements are the hardest and strongest elements.
More on Chemical Bonding:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page