Polar Molecules
![]()
Polar Molecules
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
POLAR MOLECULES
Think for a moment about the salt and sugar on your dinner They look alike. They both dissolve in water. Yet, to a they are quite different. Salt is made of ions; sodium and chlorine ions are oppositely charged and attract each. Knowing the properties of ionic substances, we know why salt is a solid. Sugar, on the other hand, is made of molecules. Molecules of sugar contains twelve atoms of carbon, twenty atoms of hydrogen, and eleven atoms of oxygen.
These atoms bonded to each other covalently. However, since a sugar is neutral, what holds sugar molecules together? Why don't the sugar molecules just float away from each other and become a gas?
Substances composed of discrete molecules exhibit a wide of melting and boiling points. There must be, therefore, a reinforces that affect the forces holding molecules to each other
14: 1 POLARITY
Recall that electronegativity is an atom's ability to attract the electrons involved in bonding. Since the electronegativity of each element differs, we should consider that in a covalent bond one of the atoms attracts the shared pair more strongly than the other. The resulting bond is said to be polar covalent.
Since one atom in the bond will attract the electrons more strongly, there will be a partial negative charge near that end of the bond.
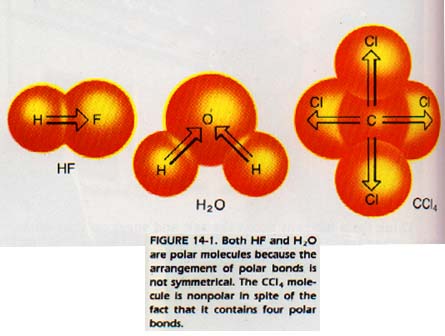
In the bond, the atom with the higher electronegativity will have a partial negative charge. The other atom will have a partial positive charge. Polar bonds, unless symmetrically arranged, produce polar molecules.
In CC14 the four C--CI bonds are polar but their symmetrical arrangement (tetrahedral) produces a nonpolar molecule.
A polar covalent bond occurs when a shared palr of electrons is attracted more strongly to one of the atoms.
Substances composed of nonpolar molecules are generally gases or low-boiling liquids. Substances composed of polar molecules generally have higher boiling points than nonpolar compounds. Many polar molecules are solids under normal conditions. A polar molecule is sometimes called a dipole. A dipole has asymmetrical charge distribution.
14:2 WEAK FORCES
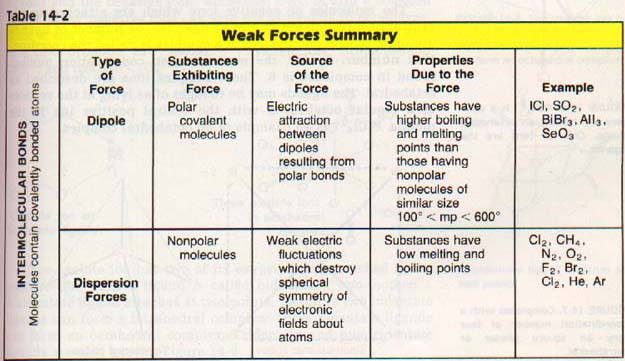
Covalent compounds show a melting point range of over 3000 Co! How can we account for such a wide variation? The forces involved in some of these cases are called van der Waals forces. Johannes van der Waals was the first to account for these forces in calculations concerning gases. These forces are sometimes referred to as weak forces because they are much weaker than chemical bonds. They involve the attraction of the electrons of one atom for the protons of another.
Weak attractive forces between the nucleus of one atom and the electrons of another atom are van der Waals forces. These are also called Intermolecular Forces.
The first source of van der Waals attraction which we will consider is dipole-dipole forces. Two molecules, of the same or different substances, which are both permanent dipoles will be attracted to each other, Figure 14-2. Such would be the case between two trichloromethane molecules CHC13 or between a trichloromethane and an ammonia molecule.

We can also have the attraction of a dipole for a molecule that is ordinarily not a dipole. When a dipole approaches a nonpolar molecule, its partial charge either attracts or repels the electrons of the other particle.
For instance, if the negative end of the dipole approaches a nonpolar molecule, the electrons of the nonpolar molecule are repelled by the negative charge. The electron cloud of the nonpolar molecule is distorted by bulging away from the approaching dipole as shown in Figure 14-3.
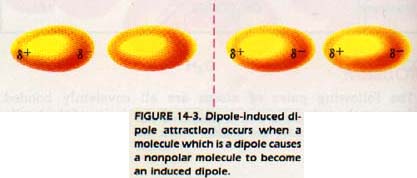
As a result, the nonpolar molecule is itself transformed into a dipole. We say it has become an induced dipole. Since it is now a dipole, it can be attracted to the permanent dipole.
Interactions such as these are called dipole-induced dipole forces. An example of this force occurs in a water solution of iodine. The I2 molecules are nonpolar while the water molecules are highly polar. The case of two nonpolar molecules being attracted must also be taken into account.
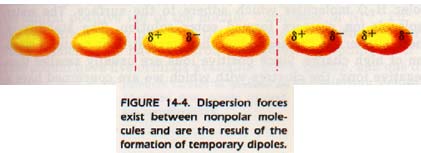
For instance, there must be some force between hydrogen molecules; otherwise it would be impossible to form liquid hydrogen. Consider a hydrogen molecule with its molecular orbital including both nuclei. We know intuitively that the electrons occupying that orbital must have a specific location. If they are both away from one end of the molecule for an instant, then the nucleus is exposed for a short time.
That end of the molecule has a partial positive charge for an instant; a temporary dipole is set up. For that time, the temporary dipole can induce a dipole in the molecule next to it and an attractive force results as shown in Figure 14-4. The forces generated in this way are called dispersion forces.
The various kinds of interactions making van der Waals forces affect each other, but we are only interested in the net result. The liquid and solid states of many compounds exist because of these intermolecular forces. These forces are effective only over very short distances. They vary roughly as the inverse of the sixth power of distance. In other words, if the distance is doubled, the attractive force is only 1/64 as large.
Of the three contributing factors to van der Waals force, dispersion forces are the most important. They are the only attractive forces that exist between nonpolar molecules. Even for most polar molecules, dispersion forces account for 85% or more of the van der Waals forces. Only in some special cases, such as NH3 and H20, do dipole-dipole interactions become more important than dispersion forces. We will examine these special cases in Chapter 17. They are called The Hydrogen Bond.
14:3 LlGANDS
As an ionic compound dissolves in water, its ions are hydrated. The surface ions of the crystal become surrounded by polar H2O molecules which adhere to the surface.
The water molecule-ion clusters formed enter solution. The stability of these clusters is greatest when they have at their center a small ion of high charge. Since positive ions are usually smaller than negative ions, the clusters with which we are concerned have a positive ion at the center. When polar molecules or negative ions cluster around a central ion, a complex ion is formed. Complex ions are widely used in analytical chemistry.
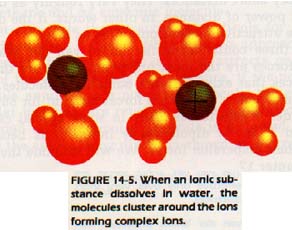
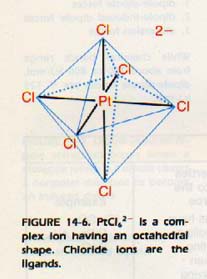
The molecules or negative ions which are attached to the central positive ion are known as ligands. The number of ligands around a central positive ion in a complex is called the coordination number. By far, the most common coordination number found in complexes is 6. These complex ions are described as octahedral. The ligands may be thought of as lying at the vertices of a regular octahedron with the central positive ion in the middle. PtC162- is an example of an octahedral complex.
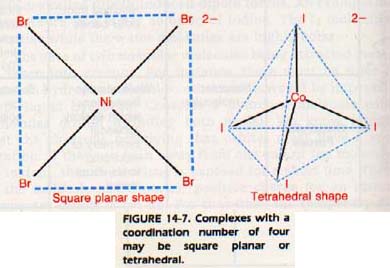
The coordination number 4 is also common. These complexes may be square-planar, with the ligands at the corners of a square and the central positive ion in the center.
Others may be tetrahedral, with the ligands at the vertices of a regular tetrahedron and the central positive ion in the middle. Complexes of Ni2+, Pd2+, Pnd Pt2+ are usually planar if the coordination number is four.
These elements all have electron configurations ending in d8. They should have five orbitals available: one s, three p, and one d. However, the d orbital turns out to be an antibonding orbital and is oriented perpendicular to the plane of the other four orbitals. An example of a square planar complex is NiBr42-
A typical tetrahedral complex is CoI42-, Figure 14-7.
The coordination number 2 is found in complexes of Ag+, Au+, and Hg2+. Complex ions with coordination number 2 are always linear. The ligands are always located at the ends and the positive ion in the middle of a straight line.
Ligands can be either molecules or negative ions. Molecular ligands are always polar and always have an unshared pair of electrons which can be shared with the central ion. The most common ligand is water. The hydrated compounds mentioned in Chapter 5 are composed of positive ions surrounded by water ligands and the negative ions. Ammonia, NH3, is also a common ligand. Many negative ions can also act as ligands in complexes.
14:8 FRACTlONATlON
Sometimes it is necessary to separate several materials from a mixture and identify them individually. A convenient method of separation based on the polarity of substances is a process called chromatography.
In chromatography, a mobile phase containing a mixture of substances is allowed to pass over a stationary phase which has an attraction for polar materials. The mobile phase consists of the mixture to be separated dissolved in a fluid (liquid or gas).
The stationary phase consists of a solid or liquid adhering to the surface of a solid. The different substances in the mixture will travel at different rates due to their varying polarity.
There are several polarity considerations in determining the rate at which each component of the mixture will migrate. A polar substance will have some attraction for the solvent as well as an attraction for the stationary phase. The stationary phase will attract some of the substances more strongly than others.
The slowest migrating substance will be the one with the greatest attraction for the stationary phase. The fastest migrating substance will be the one with the least attraction for the stationary phase. Thus, they can be separated.
These separations are called fractions because they are parts of the whole. The overall separation of parts from a whole by any process may be called fractionation.
Often, the fractions are different colors. For this reason, this separation method is called chromatography (writing with color). Gas chromatography does not involve color. However, since it also depends upon fractionation, the name chromatography is still applied.
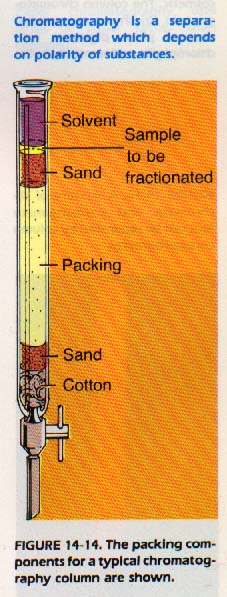
14:9 COLUMN CHROMATOGRAPHY
In column chromatography, a glass column is used to carry out the separation. The glass tube is packed with a stationary phase such as calcium carbonate. Other packings now in use are magnesium or sodium carbonate, activated charcoal, ion exchange resins, clays, gels, and many organic compounds.
The mobile phase with the material to be fractionated is added to the top of the column. Then fresh solvent is caused to percolate through the column. The solvent may be water, ammonia, an acid, an alcohol, or other organic or inorganic substance.
In column chromatography a solvent percolates through a packings substance.
Each substance in the mobile phase migrates down the tube at a different rate and the substances are separated. The rate of travel depends upon the attraction of each substance for the stationary phase, its attraction for the solvent, and the solvent concentration. If the substance has a high attraction for the stationary phase, only a high concentration of solvent will dislodge it.
As the solvent moves down the tube, it becomes less concentrated, and the solute becomes more attracted to the stationary phase again. Those substances with less attraction for the stationary phase are carried farther, even by the less concentrated solvent.
By constant percolation, the substances are separated into zones. A sample chromatogram is shown in Figure 14-16. In the column shown, dichromate ions and permanganate ions are being separated on a packing of aluminum oxide.
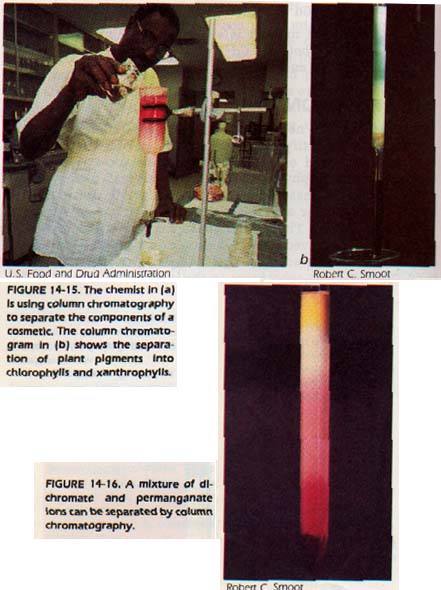
The solvent being used is dilute nitric acid. As you can see, the dichromate is more strongly attracted to the stationary phase so it moves down the column more slowly.
After separation, it may be necessary to recover the material in each separate zone for identification. One method involves forcing the zones out the bottom of the tube, one at a time. The substance in each zone is dissolved and then recovered by evaporation.
A second method uses solvents of increasing polarity until each zone comes out the bottom along with the solvent. Identification is then made by any number of methods. Column chromatography is used now for extremely delicate separations. Complex substances such as vitamins, proteins, and hormones, not easily separated by other methods, can be separated by chromatography.
14:10 SURFACE CHROMATOGRAPHY
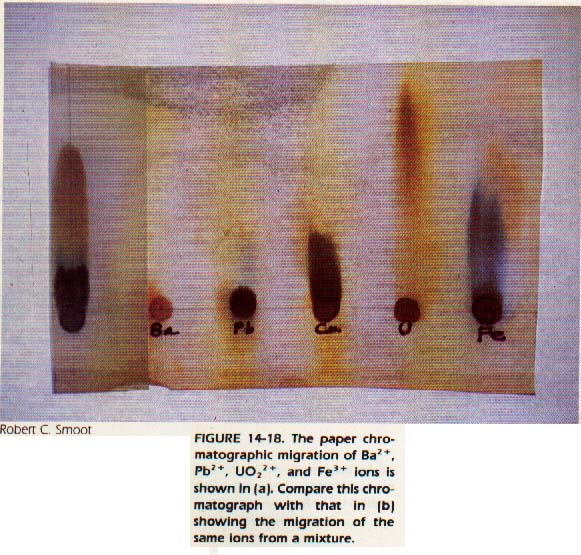
Paper chromatography is a form of chromatography in which the separations are carried out on paper rather than in glass columns.
Strips of paper are placed in a box or bell jar in which the atmosphere is saturated with water vapor or solvent vapor. A drop of the solution to be separated is placed at the top of the paper. The paper is then overlapped into solvent at the top of the box.
The solvent moves down the paper by capillary action, separating the constituents of the drop. The paper may be placed into solvent at the bottom of the box. In this case, the drop of the solution would be placed at the bottom of the paper and the solvent would ascend the paper. In either case, the separations are seen as a series of colored spots on the paper strip.
If the separated fractions happened to be colorless, they can be sprayed with solutions which will produce colored compounds. Some of these compounds may fluoresce under ultraviolet light.
Paper chromatography is simple, fast, and has a high resolving power. This method can be used to separate the constituents of blood, urine, and antibiotics.
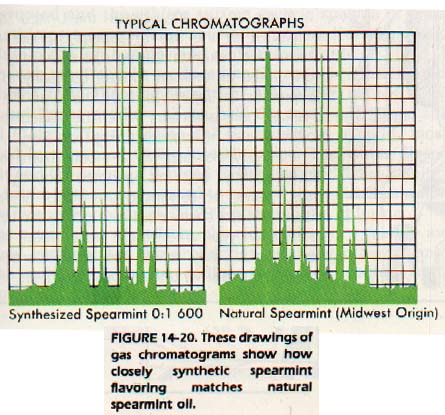
1 4: 1 1 GAS CHROMATOGRAPHY
Volatile liquids and mixtures of gases or vapors can also be analyzed by a chromatographic process. The gases to be analyzed are carried along by an inert gas such as helium in the mobile phase. The gases are fractionated on a stationary phase by a method similar to column chromatography. They are then carried by the inert helium through a tube fitted with an electrocouple. The varying amounts of contamination in the helium produce varying amounts of current. These variations are recorded by a needle on a moving sheet of graph paper. The amount of gas present in each fraction can be determined from the area under the curve produced.
SUMMARY
1. A polar bond is one in which a shared pair of electrons is attracted more strongly to one of the atoms.
2. van der Waals forces are the net result of dipole-dipole, dipole-induced dipole, and dispersion effects.
3. A complex ion is composed of a central positive ion and molecular or negative ion ligands.
4. The number of ligands surrounding the central ion is the coordination number of a complex.
5. Complex ions which contain more than one type of ligand may exhibit geometric isomerism.
6. Small positive ions with a large nuclear attractive force form excellent central ions. The transition metal ions are good examples of these.
7. The bonds of complex ions have both ionic and covalent bonding characteristics.
8. Chromatography is a method of separating substances into identifiable chemical fractions by differences in their polarity.
More on Polar Molecules:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page