The Mole
![]()
The Mole
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
THE MOLE
Chemical symbols and formulas (such as H and H20) are shorthand signs for chemical elements and compounds. The symbol of an element may represent one atom of the element. The formula of a compound may represent one molecule or one formula unit of the compound. Symbols and formulas may also represent a group of atoms or formula units. Since atoms are so very small, chemists deal with large groups of atoms. This chapter is about a group called a mole, containing a specific number of units.
5:1 MOLECULAR MASS
The masses of the atoms are compared by using the atomic mass scale. This scale has the "atomic mass unit" (amu) as a standard. The source of this standard will be discussed in Chapter 8. Here is a list of atomic masses for the elements:
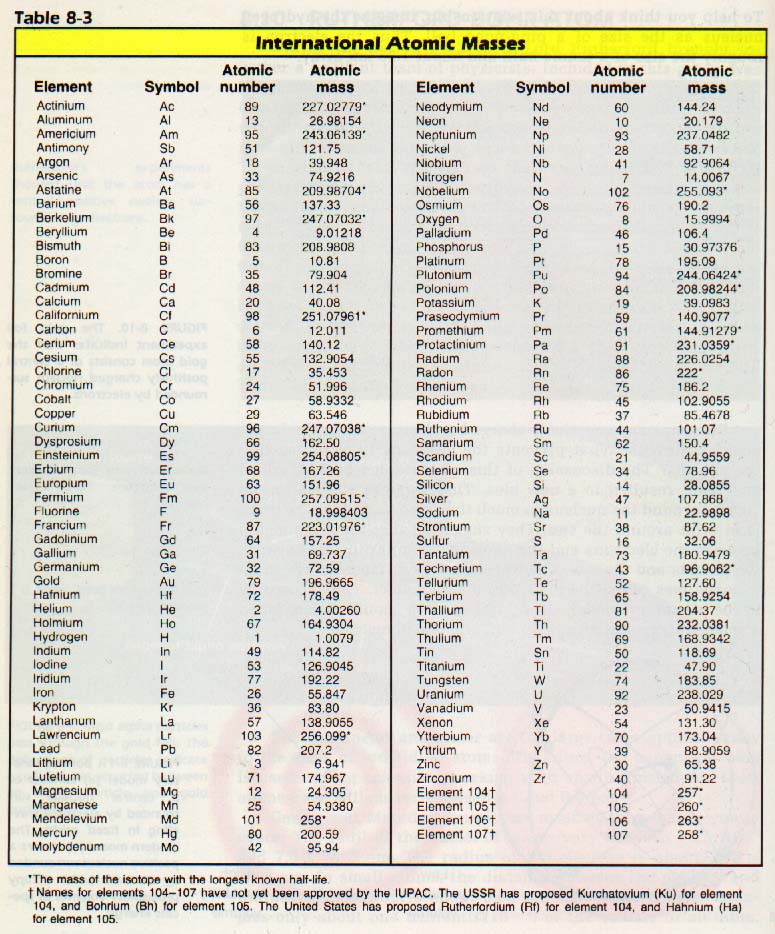
The atomic mass of hydrogen in atomic mass units is 1, and the atomic mass of oxygen is 16. Therefore, the total mass of a water molecule, H2O, is 1+1+16, or 18 amu.
If the atomic masses of all the atoms in a molecule are added, the sum is the mass of that molecule. Such a mass is called a molecular mass.
This name is incorrect when applied to an ionic substance. Sodium chloride, NaCl, is an ionic substance which does not exist in molecular form. A better name for the mass of ionic substances is formula mass. The sum of the atomic masses of all atoms in the formula unit of an ionic compound is called the formula mass of the substance.
EXAMPLE: Formula Mass
Find the formula mass of sodium sulfate, Na2SO4.
Solving process:
Add the atomic masses of all the atoms in the Na2SO4 unit.
2 Na atoms, 2 x 23 = 46 amu
1 S atom, 1 x 32 = 32 amu
4 O atoms, 4 x 16 = 64 amu
The total formula mass = 142 amu
EXAMPLE: Formula Mass
Find the formula mass of calcium nitrate, Ca(NO3)2
Solving process:
Add the atomic masses of all the atoms in the Ca(NO3)2 formula unit. Remember that the subscript applies to the entire polyatomic ion.
1 Ca atom, 1 x 40 = 40 amu
2N atoms, 2 x 14 = 28 amu
6 O atoms, 6 x 16 = 96 amu
Total formula mass is 164 amu
5:2 AVOGADRO'S NUMBER
There is one problem in using the molecular masses of substances. These masses are in atomic mass units. An atomic mass unit is only 1.66 x 10-24 gram. The mass of a single molecule is so small that it is impossible to measure it in the laboratory. For everyday use in chemistry, a larger unit, such as a gram, is needed.
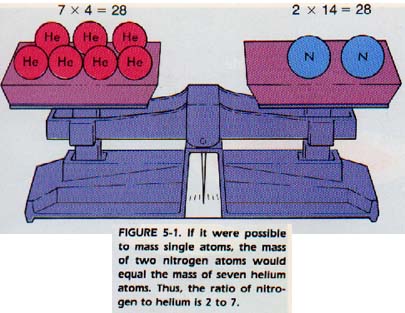
One helium atom has a mass of 4 amu, and one nitrogen atom has a mass of 14 amu. The ratio of the mass of one helium atom to one nitrogen atom is 4 to 14, or 2 to 7.
Let us compare the mass of two helium atoms to that of two nitrogen atoms. The ratio would be 2 x 4 to 2 x 14, or 2 to 7. If we compare the mass of 10 atoms of each element, we will still get a 2 to 7 ratio. No matter what number of atoms we compare, equal numbers of helium and nitrogen atoms will have a mass ratio of 2 to 7. In other words, the numbers in the atomic mass table give us the relative masses of the atoms of the elements.
The laboratory unit of mass you will use is the gram. We would like to choose a number of atoms which would have a mass in grams equivalent to the mass of one atom in atomic mass units. The same number would fit all elements since equal numbers of different atoms always have the same mass ratio.
Chemists have found that 6.02 x 1023 atoms of an element have a mass in grams equivalent to the mass of one atom in amu.
For example, one atom of hydrogen has a mass of 1.0079 amu; 6.02 x 1023 atoms of hydrogen have a mass of 1.0079 g. This number, 6.02 x 1023 is called Avogadro's Number in honor of a 19th century Italian scientist.
5:3 THE MOLE
Avogadro's number is an accepted SI standard. We may add it to the others we have studied: the kilogram, the meter, the second, and the kelvin.
The symbol used to represent Avogadro's number is N,. This quantity can be expressed as 6.022 17 x 1023 to be more precise. This number of things is called one mole (mol) of the things. Recall that the mole is an SI base unit representing the amount of substance.
One mole of particles (atoms, ions, molecules) has a mass in grams equivalent to that of one particle in atomic mass units. Thus, if a mole of any particle has a mass of 4.02 grams, then a single particle has a mass of 4.02 amu.
In the same manner, if a single particle has a mass of 54.03 amu, then a mole of particles will have a mass of 54.03 grams. NA is a number that has been experimentally determined.
If a mole of iodine molecules is inspected by X-ray diffraction, the actual number of I2 molecules can be determined. This number has been found to be 6.02 x 1023 molecules.
Avogadro's number has also been determined by the scattering of light, by use of radioactive materials, and by Millikan's oil drop experiment which is covered in Chapter 8.

It is important to note that one mole of atoms contains 6.02 x 1023 atoms. One mole of molecules contains 6.02 x 1023 molecules. One mole of formula units contains 6.02 x 1023 formula units. One mole of ions contains 6.02 x 1023 ions. NA , therefore, can have any of these units:
molecules / mole
atoms / mole
ions / mole
EXAMPLE: Conversion to Moles
How many moles are represented by 11.5 grams of C2H5OH?
Solving process:
We have grams, we wish to convert to moles. Using the atomic mass table above, we see that one mole of C2H5OH has a mass of 46.1 grams. So divide the number of grams by the molecular mass.
Click on these links for more on mole problems:
.......... The Mole
.......... The Size of the Mole
.......... Rules for Problem Solving
5:4 MOLES IN SOLUTlON
Many, if not most, chemical reactions take place in water solution. There are several methods of expressing the relationship between the dissolved substance and the solution. The method most often used by chemists is molarity (M). Molarity is the ratio between the moles of dissolved substance and the volume of solution in cubic decimeters (liters). Remember that 1 dm3 = 1 L = 1OOO cm3.
A one-molar (1M) solution of nitric acid contains one mole of nitric acid molecules in one dm3 (liter) of solution. A 0.372M solution of Ba(N03)2 contains 0.372 moles of Ba(N03)2, in 1 dm3 (liter) of solution.
You can see that expressing the composition of solutions in units of molarity is a convenient way of measuring a number of particles.
It is important to be able to compute the molarity of solutions if you are given their composition. It is also important to be able to compute the amount of substance you need to produce a specific solution. You will be using these calculations in your laboratory work and later in solving problems in this book.
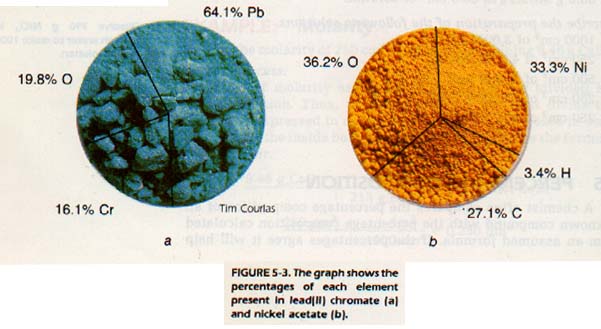
5:5 PERCENTAGE COMPOSITION
A chemist often compares the percentage composition of an unknown compound with the percentage composition calculated from an assumed formula. If the percentages agree it will help confirm the identity of the unknown.
The percentage of the total mass of a compound contributed by an element is the percentage of that element in the compound. Consider the percentage composition of the following substances: copper, sodium chloride, and ethanol.
For copper, the percentage composition is 100 percent Cu because it is composed of a single element.
Salt is composed of two elements, sodium and chlorine. We know they are always present in the same ratio by mass. The ratio in which they are present is the ratio of their atomic masses. Therefore, the percentage of sodium in any sample of sodium chloride would be the atomic mass of the element divided by the formula mass and multiplied by 100.
5:6 EMPlRlCAL FORMULAS
Using experimental data we can find the empirical formula for a substance. We need only know the mass of each element in the substance. Elements are made of atoms, and compounds are made of elements. Half an atom does not exist.
Therefore, we can state that the elements in a compound combine in simple whole number ratios, such as 1 to 1, 1 to 2, 2 to 3, and so on. If the atoms of the elements are present in simple ratios, then the moles of atoms for each element in the substance will be in small whole number ratios.
Consider the following example. We find a 2.5-gram sample of a certain substance contains 0.9 gram of calcium and 1.6 grams of chlorine. The substance is composed of only two elements.
We can calculate the number of moles of calcium and the number of moles of chlorine in the compound. Then, we can find the ratio of the number of moles of calcium atoms to the number of moles of chlorine atoms.
From this ratio, we can find the empirical formula, which is the simplest ratio of atoms in a compound.
5:7 MOLECULAR FORMULAS
We have, thus far, calculated empirical formulas from experimental data. In order to calculate a molecular formula, we need one additional piece of data, the molecular mass.
In one of the examples in the previous section, the empirical formula calculated was CH2O. If we know that the molecular mass of the compound is 180 g/mol, how can we find the molecular formula?
The molecular formula shows the number of atoms of each element in a molecule. Knowing that the elements will always be present in the ratio 1:2:1, we can calculate the mass of the empirical formula. Then we can find the number of these empirical units present in one molecular formula. In the substance CH20, the empirical unit has a mass of
12 + 2(1) + 16, or 30.
It will, therefore, take six of these units to equal 180 or one molecular formula. Thus, the molecular formula is C6H1206.
5:8 HYDRATES
There are many compounds which crystallize from a water solution with water molecules adhering to the particles of the crystal. These hydrates, as they are called, usually contain a specific ratio of water to compound.
Chemists use heat to dry these compounds and then calculate the ratio of compound to water. An example of a hydrate is NiSO3 • 6H2O. The dot shows that 6 molecules of water adhere to 1 molecule of formula unit.
To calculate the formula mass, we add the formula mass of the compound and water. For NiSO3 , we obtain 139 g/mol. We multiply the 18 for water by 6 and add to the 139. The formula mass of NiSO3 • 6H2O is then 139 + 6(18), or 247.
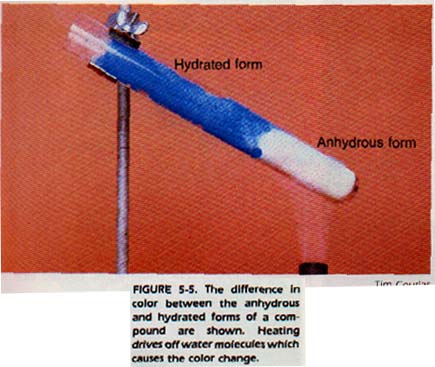
Hydrate means it has loooosley bonded water molecules
Anhydrous means there is no water of hydration attached.
SUMMARY
1. The symbol for an element represents one atom of the element or one mole of the element when it stands alone.
2. The formula for a compound can represent one molecule or formula unit. It can also represent one mole of the compound.
3. Masses of atoms are based on the atomic mass unit (amu).
4. The molecular mass of a molecule is found by adding the atomic masses of all the atoms in one molecule.
5. Not all substances exist normally as molecules. Therefore, the term molecular mass is not used for all substances. The mass of the empirical formula unit of an ionic substance such as sodium chloride is called the formula mass.
6. There are 6.02 x 1023 particles in 1 mole. This quantity is known as Avogadro's number. The number of moles in a given mass of substance can be found by dividing the total mass by the formula mass expressed in units of g/mol.
8. The percentage of the total mass of a compound contributed by an element is the percentage of that element in the compound.
9. The empirical formula indicates the simplest whole number ratio of atoms present in a compound.
10. The molecular formula of a compound is some whole number multiple of the empirical formula.
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
 Return to the Big Chem Page
Return to the Big Chem Page