Solutions
![]()
Solutions
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
SOLUTIONS
What do we mean by solution? In Chapter 3, we referred to heterogeneous matter and homogeneous matter. Homogeneous matter is the same throughout. It is often made of only one substance-- a compound or an element. Homogeneous matter may also be made of a mixture of several substances. Such a homogeneous mixture is called a solution. Most, but not all, solutions consist of a solid dissolved in a liquid. The particles in a true solution are molecules, atoms, or ions which will pass easily through the pores of filter paper. Solutions cannot be separated into their components by filtration.
The substance which occurs to the greater extent in a solution is said to do the dissolving and is called the solvent. The less abundant substance is said to be dissolved and is called the solute.
21:1 THE SOLVENT
The most common solvent is water. Using water as a typical solvent, let us look at the mechanism of solvent action. Water molecules are very polar. Because they are polar they are attracted to other polar molecules and to ions.
Table salt, NaCl, is an ionic compound made of sodium and chloride ions. If a salt crystal is put in water, the polar water molecules are attracted to ions on the crystal surfaces. The water molecules gradually surround and isolate the surface ions. The ions become hydrated, Figure 21-1.
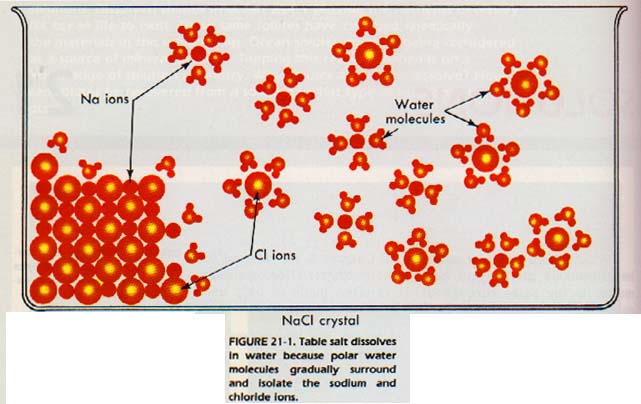
The attraction between the hydrated sodium and chloride ions and the remaining crystal ions becomes so small that the hydrated ions are no longer held by the crystal. They gradually move away from the crystal into solution. This separation of ions from each other is called dissociation. The surrounding of solute particles by solvent particles is called solvation.
The dissociation of ions in solution leads to a factor which is important to keep in mind any time you are working with a solution of an ionic material. When the ions are dissociated, each ion species in the solution acts as though it were present alone. Thus, a solution of sodium chloride acts as a solution of sodium ions and chloride ions.
There is no characteristic behavior of "sodium chloride" in solution because there really is no sodium chloride in solution. There is simply a solution containing both sodium ions and chloride ions uniformly mixed.
21:2 SOLVENT-SOLUTE COMBlNATlONS
Four simple solution situations can be considered. They are listed in Table 21-1. Not all possible combinations of substances will fit into these four rigid categories. However, we will now consider these four as sample cases for studying solutions.
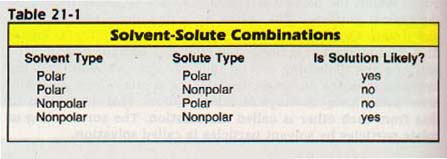
(1) Polar solvent-Polar solute.
The mechanism of solution involving a polar solvent and a polar solute is the one we have already described for salt and water. The polar solvent particles solvate the polar solute particles. They attach themselves due to the polar attraction. The intracrystalline forces are reduced so much that the surface particles are carried away by the solvent particles. In water, this process is called hydration.
(2) Polar solvent-Nonpolar solute.
Because these solvent particles are polar, they are attracted to each other. However, the solute particles in this case are nonpolar and have little attraction for particles of the solvent. Thus, solution to any extent is unlikely, as we see if we try to dissolve wax in water.
(3) Nonpolar solvent-Polar solute.
Reasoning similar to that of the second case applies here. The solvent particles are nonpolar and thus have little attraction for the solute particles. In addition, the solute particles in this case are polar and are attracted to each other. Again, solution to any extent is unlikely, as we see if we try to dissolve salt in gasoline.
(4) Nonpolar solvent-Nonpolar solute.
Only van der Waals forces (intermolecular) exist among the nonpolar solvent particles. The same is true for the nonpolar solute particles. Thus, all particles in the solution are subject only to van der Waals forces, and solution can occur. Random motion of solute molecules will cause some of them to leave the surface of the solute. There can be solvation in such cases, but the forces involved are far weaker than those in solutions involving polar compounds. The nonpolar particles are simply randomly dispersed. Thus, wax will dissolve in gasoline.
Not all nonpolar substances are soluble in each other, however. Let us consider the most common type of solution: A solid dissolved in a liquid. The solubility of nonpolar solid in a nonpolar liquid depends upon two factors. These factors are its melting point and its heat of fusion.
What do they have to do with solubility? When the solid dissolves, a liquid solution results. The solid is undergoing a phase change. Solids with low melting points and low heats of fusion will be more soluble than those with high melting points and high heats of fusion.
21:3 SOLIDS, LIQUIDS, AND GASES IN SOLUTlON
Since there are three common physical states of matter (solid, liquid, and gas, there are nine possible combinations of solvent solute pairs. These combinations are given below. Table 21-3

The property of mutual solubility of two liquids is called miscibility. If two liquids are mutually soluble in all proportions, they are said to be completely miscible. Ethylene glycol (automobile antifreeze) and water are two such liquids. Water and carbon tetrachloride, however, do not appreciably dissolve in each other and are, therefore, immiscible. Two liquids such as ethyl ether and water, which dissolve in each other to some extent but not completely, are referred to as partially miscible.
A number of metals, such as gold and silver, are mutually soluble and form solid--solid solutions. Such solid metal--metal solutions constitute one type of alloy.

2l:4 SOLUTlON EQUlLlBRlUM
When crystals are first placed in a solvent, many particles may leave the surface and go into solution. As the number of solute particles in solution increases, some of the dissolved particles return to the surface of the crystal.
Eventually the number of particles leaving the crystal surface equals the number returning to the surface. This point is called solution equilibrium.
At a specific temperature, there is a limit to the amount of solute that will dissolve in a given quantity of solvent. For instance, at 20oC, A maximum of 64.2 grams of nickel chloride will dissolve in 100 cm3 of water. This quantity is called the solubility of the substance at 20oC.
This solubility can be changed by altering the temperature. A solution in which undissolved substance is in equilibrium with the dissolved substance is called a saturated solution. A solution containing less than the saturated amount of solute for that temperature is an unsaturated solution.
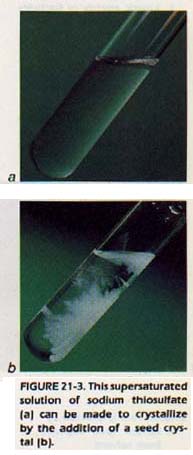
Larger amounts of solute can usually be dissolved in a solvent at a temperature higher than room temperature. If the hot solution is then cooled, an unstable solution is formed. This solution contains more solute than a saturated solution can hold. The solution is called a supersaturated solution.
Supersaturation is possible because solids will not crystallize unless there is a special surface upon which to start crystallization. A container which has a smooth interior and which contains a dust-free solution has no such surfaces. However, a supersaturated solution will crystallize almost instantly if a crystal of the salute is introduced. How could you find out if a solution is saturated, unsaturated, or supersaturated?
Precipitation reactions are an important tool of the analytical chemist. The differing solubilities of substances can be used to separate them from mixtures. If a solution contains two ionic compounds, they may be separated by choosing a reactant that will precipitate only one ion.
For example, consider a mixture of sodium nitrate and barium chloride. A chemist wishes to know what percentage of the mixture is BaC12, and thus finds the mass of the mixture before dissolving it in water.
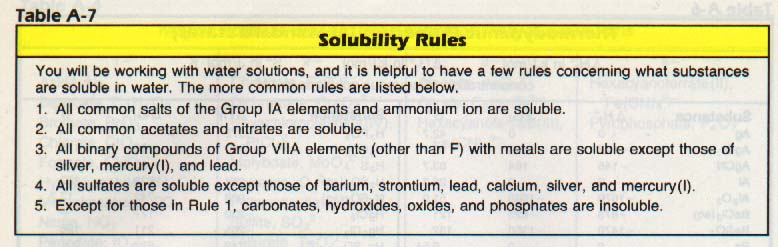
Once dissolved, the barium ions could be precipitated with sulfate ions from a soluble sulfate such as sulfuric acid. On the other hand, the chloride ions could be precipitated with silver ions from a soluble silver salt such as silver nitrate (see Table A-7). The product is collected, dried, and massed. By solving a mass-mass problem, the chemist can find the amount, and thereby the percentage of BaCl2 in the original mixture.
21:5 SOLUTlON RATE
The rate of solution is affected by the surface area of the crystal which is exposed to fresh solvent. When the area of an exposed surface is increased, more solute particles are subjected to solvation.
The surface area can be increased by breaking the crystal to be dissolved into very small particles. The surface area can also be increased by stirring the mixture as the solute is dissolving.
In this way, the solvent which is saturated with solute is moved away from the surface of the solid solute. Fresh solvent can then come into contact with the solid surface. Solution rate is also a function of the kinetic energy of both the solute and solvent particles. The faster the solvent particles are moving, the more rapidly they will circulate among the crystal particles.
This motion has the same effect as increasing the surface area. If a solute particle's kinetic energy is increased, once it is solvated, it will move away from the solid material more rapidly.
This motion exposes fresh surface, thus increasing solution rate. Finally, with increased kinetic energy, particles are more easily removed from the crystal. The kinetic energy of a system is increased by heating the mixture.

21:6 HEAT OF SOLUTION
The reaction involving the solution of most solids in water is endothermic, ΔH positive. Recall from Chapter 20 that the free energy difference, ΔG, for a change is negative if the reaction proceeds spontaneously. For ΔG to be negative in the equation ΔG = ΔH - TΔS, ΔS must be positive. If ΔH is positive, the solid has a high degree of order in the crystalline form.
When dissolved, the particles are randomly distributed throughout the solution. The random distribution represents more disorder than the crystal. We see, then, that ΔS is positive, as predicted.
When gases dissolve in water, the hydrated molecules represent a higher degree of order than the random distribution in the gas. ΔS for the dissolving of a gas is, therefore, negative.
Soluble gases, then, must have a negative ΔH. Experiment shows that the dissolving of gases is, in fact, an exothermic process. There are a few solids with a negative ΔH of solution. For these substances the degree of hydration is so high that more order exists in the solution than in the separate solid and liquid.
Using our knowledge of enthalpy change in solutions, we can predict the effect of temperature on solubility. Most solids having positive heats of solution, ΔH> 0, are more soluble in hot water than in cold. However, gases, with negative heats of solution, ΔH< 0, are more soluble in cold water.
21:7 EFFECTS OF PRESSURE
Pressure has little effect on solutions unless the solute is a gas. The amount of gas which dissolves in a given amount of solvent is greater at high pressure than it is at low pressure.
The mass of a gas which will dissolve in a liquid at a given temperature varies directly with the partial pressure of that gas. This statement is Henry's Law, named in honor of William Henry, the English chemist who first discovered this relationship.
21:8 SOLUTlON PROPORTlONS
Solutions may also be classified on the basis of the amounts of solvent and solute present. If a relatively large of solute is present per unit volume, the solution is a solution. If only a relatively small amount of the solute is per unit volume, the solution is a dilute solution.
This terminology is less exact than indicating the degree of saturation or the ratio of solute to solvent.
21:9 MOLAR SOLUTlONS
In Chapter 5 we learned about a precise concentration unit called molarity. We found that a one-molar, 1M, solution contains 1 mole of solute in 1 dm3 (or liter) of solution. If 1 mole of sodium chloride is dissolved in enough water to make 1 dm3 of solution, the solution is a 1-molar solution of sodium chloride. Sodium chloride is in the form of dissociated ions in solution. Therefore, the solution can also be said to be l-molar in sodium ions and 1-molar in chloride ions.
Molarity is the most common concentration unit in chemistry. Measurement of the volume of solutions is fast and convenient. If the solution measured has a known molarity, a measurement of volume is also a measurement of a number of particles.
Each unit of volume contains a known number of ions or molecules. Multiplying the concentration in molarity (moles per dm3) by the volume (dm3) will give you the number of particles (in moles). Whenever the concentration of solute in a solution is known with some degree of precision, the solution is called a standard solution.
SUMMARY
1. Polar solvents tend to dissolve polar solutes; nonpolar solvents tend to dissolve nonpolar solutes.
2. When ionic compounds dissolve, the ions dissociate.
3. A solution is said to reach solution equilibrium when the rates of leaving and returning to the solution are equal. When solution equilibrium is reached, the solution is said to be saturated with the solute.
4. If a relatively large amount of solute is present per unit volume, the solution is a concentrated solution.
5. The rate of solution is affected by the surface area of crystal exposed and the kinetic energy of solute and solvent.
6. Heat of solution is the enthalpy change which occurs when one substance is dissolved in another. Most solids have positive heats of solution. Gases have negative heats of solution.
7. Henry's law: The mass of a gas which will dissolve in a liquid at a given temperature varies directly with the partial pressure of that gas.
8. Molarity is a common concentration unit.
More on Solutions:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page