Kinetic Theory
![]()
Kinetic Theory
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
KINETlC THEORY
The kinetic theory explains the effect of heat and pressure matter. First, let us consider some basic assumptions of the theory. All matter is composed of particles (atoms, ions, or molecules). These small particles are in constant motion. Finally, collisions are perfectly elastic.
Perfectly elastic means that there is no change in the total kinetic energy of two particles had before after their collision. It may be difficult to imagine that particles in a great structure such as the Golden Gate Bridge are in constant motion.
However, as we shall see, many of the properties of matter are the result of such motion. As early as the mid seventeenth century, the English inventor Robert Hooke proposed a kinetic theory. He predicted that there were particles nature which were in constant motion. In order to have some concept of the size for the quantities involved, we will first discuss oxygen.
15:1 OXYGEN MOLECULES IN MOTlON
At room temperature (25oC), the average velocity of an oxygen molecule is 443 m/s. This speed is equivalent to just over 1700 kilometers per hour.
At this speed, the molecules collide with othermolecules frequently. We can determine the average number of collisions a molecule undergoes in a unit of time. We do so by the average distance a molecule travels before colliding with another molecule.
This distance is called the mean free path of the molecule. For oxygen at 25oC, the mean free path is 106 nm. The diameter of the oxygen molecule is about 0.339 nm. Thus an oxygen molecule at 25oC will travel, on the average, about 314 times its own diameter before colliding with another iinolecule.
This travel corresponds to a little over four and a half billion collisions per second per molecule.
We give these figures for oxygen as examples of the speed, the distance of travel, and the number of molecular collisions in a gas. These factors vary with the temperature and the mass of the particles composing the gas.
15:2 PRESSURE
Besides colliding with each other gas molecules collide with the walls of the container in which the gas is confined. When a gas molecule collides with the wall of a container, it exerts a force on the container. It is the force of collision and the number of collisions with the walls of a container that cause gas pressure, Figure 15-2. This pressure is measured in terms of the force per unit area.
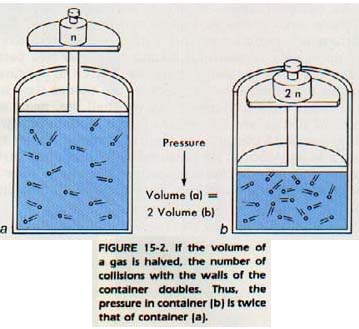
The molecules and atoms of the gases present in the air are constantly hitting the surface of the earth and everything on it. As a result, you and everything surrounding you are subject to a certain pressure from the molecules of the air.
Air pressure has been used as a scientific standard of pressure. The standard is defined as the average pressure of the air at sea level under normal conditions. Since conditions in the air depend upon many weather factors, it is difficult to define "normal" conditions at sea level.
Therefore, the standard has been defined in terms of a system which can be reproduced in the laboratory. It is 101.325 kilopascals (kPa). One pascal is a pressure of one newton per square meter (N/m2). We will generally employ units of kilopascals.
15:3 MEASURlNG PRESSURE
In measuring gas pressure, an instrument called a manometer is used. Two types of manometers are shown Figure 15-3. In the "open" type, air exerts pressure on the of liquid in one arm of the U-tube. The gas being studied pressure on the other arm. The difference in liquid level the two arms is a measure of the gas pressure relative the air pressure. If you know the density of the liquid in the you can calculate the pressure difference between the gas and the air.
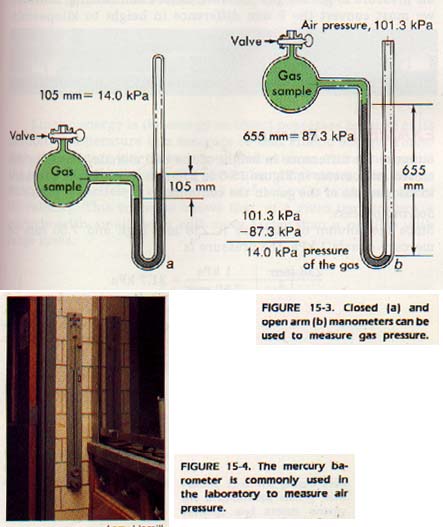
The "closed" type of manometer has a vacuum above the liquid in one arm. The pressure measured with an instrument of this type does not depend on the pressure of the air and is called the absolute pressure. This manometer can be used to measure the pressure of the air itself. Such a manometer (one used to ineasure atmospheric pressure) is called a barometer.
Most barometers are manufactured with a scale calibrated to read the height of a column of mercury in millimeters. The average pressure of the air will support a column of mercury 760 mm high. Since average air pressure is defined as 101 kPa, then 101 kPa = 760 mm. By dividing both sides of the equation by 101 we find that
1 kPa = 7.50 mm of Hg
so 1 mm = 1.33 kPa
Even with an open manometer it is possible to calculate the absolute pressure of a gas. However, a barometer must be available to measure the atmospheric pressure on the outside arm of the manometer.
15:4 KlNETlC ENERGY
The average speed of the particles in a gas depends only on temperature and the mass of the particles. How are temperature and particle mass related?
Kinetic energy is the energy an object possesses because of its motion. Temperature is a measure of that kinetic energy.
Therefore, the average kinetic energy of molecules or atoms in a gas is the same for all particles at a particular temperature. The kinetic energy of a particle is equal to 1/2 mv2, where m is its mass and v is its velocity. This equation shows that, at a given temperature, a particle with small mass will move faster than a particle with large mass.
15:5 TEMPERATURE
A decrease in the temperature of a substance means that the particles of the substance are moving more slowly. An increase in temperature means the particles are moving faster. Temperature is a measure of the average kinetic energy of the particles of a substance.
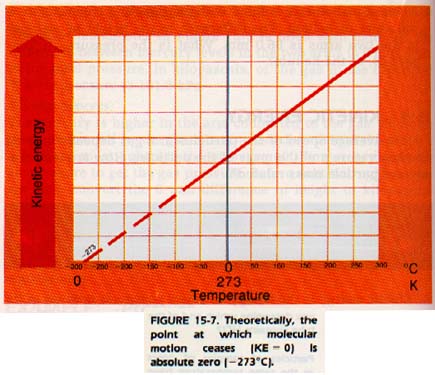
Molecular motion of a gas decreases as the temperature decreases.
Therefore, it should in theory be possible to lower the temperature to a point where all molecular motion ceases. This temperature, the temperature at which all molecular motion should cease, is known as absolute zero. It is -273oC.
To make a temperature scale based on absolute zero, scientists have agreed on a system known as the absolute, or kelvin scale. The zero point of the kelvin scale is absolute zero. The divisions, or degrees, are the same size as those of the Celsius scale. Therefore, the kelvin is the SI unit of temperature
K = oC + 273o
15:6 HEAT TRANSFER
Temperature can be used to determine the direction of flow of energy. Heat enery always flows from a warmer object cooler one. Kinetic theoIy explains the flow of heat energy in terms of particle collisions. The particles of the warmer object and the cooler object have unequal kinetic energy.
The excess energy of the particles in the warmer object is passed on to the particles of the cooler object as they collide.
15:7 STATES OF MATTER
Matter exists in four states--solid, liquid, gas and plasma. Thus far, our discussion of the kinetic theory has been limited to gases. However, kinetic theory can also be used to explain the behavior of solids and liquids. Plasmas are treated as a special case.
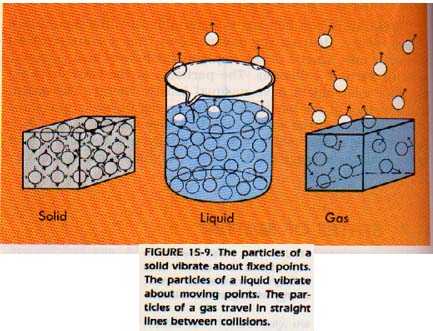
Gas particles are independent of each other and move in a straight line. Change of direction occurs only when one particle collides with another, or when a particle collides with the walls ofthe container. Gas particles, then, travel in a completely random manner.
Since they travel until they collide with a neighbor or with the walls of their container, gases assume the shape and volume of their container.
The particles of a liquid have what appears to be a vibratory type of motion. Actually, they are traveling a straight-line path between collisions with near neighbors. The point about which the seeming vibration occurs often shifts as one particle slips past another.
These differences in the amount of space between particles allow the particles to change their relative positions continually. Thus, liquids, although they have a definite volume, assume the shape of their container.
In solids, a particle occupies a relatively fixed position in relation to the surrounding particles. A particle of a solid appears to vibrate about a fixed point. Again, the particle is actually traveling a straight-line path between collisions with very near neighbors.
For example, a molecule of oxygen gas at 25oC travels an average distance equal to 314 times its own diameter before colliding with another molecule. In a solid, however, the particles are closely packed and travel a distance equal to only a fraction of their diameters before colliding. Unlike liquids, solids have their particles arranged in a definite pattern. Solids, therefore, have both a definite shape and a definite volume.
The physical state of a substance at room temperature and standard atmospheric pressure depends mostly on the bonding in the substance. Ionic compounds have strong electric charges holding the ions together and exist as solids.
Molecular substances are attracted to each other by van der Waals forces. Molecular compounds of high molecular mass tend to be solids.
Nonpolar molecules of low molecular mass tend to be gases. Greater molecular mass and greater polarity both tend to make substances form a condensed state, either liquid or solid.
The arrangement of particles is important to the chemist. In the following chapters we will discuss some of the special characteristics of solids, liquids, and gases.
15:8 PLASMA
When matter is heated to very high temperatures (>5000oC), the collisions between particles are so violent that electrons are knocked away from atoms. Such a state of matter, composed of electrons and positive ions, is called a plasma.
Most of the universe is made of plasma. Stars are in a plasma state. Outer space is not really empty. It is composed of extremely thin plasma. The Van Allen radiation belts which surround the earth are made of plasma. Matter in a neon tube or in a cyclotron is in the form of plasma.
Scientists are working on the character of plasmas because a nuclear reaction, called fusion, occurs only in plasmas. The fusion reaction, if it can be controlled, promises to be an energy source second only to the sun.
Since plasma consists of charged particles traveling at high speeds, it is greatly affected by electric and magnetic fields.
Therefore the study of plasma is called magnetohydrodynamics (MHD) is involved in confining the plasma which scientists hope can be used as an energy source through nuclear fusion reactions. It isalso involved in designing an advanced propulsion unit for space
SUMMARY
1. The kinetic theory explains the effects of heat and pressure on matter. This theory assumes that (1) all matter is composed of particles; (2) these particles are in constant motion; (3) collisions between these particles are perfectly elastic.
2. At 25oC, an oxygen molecule travels at just over 1700 kilometers per
hour in straight lines. At this temperature it has more than four and a half billion collisions per second.
3. A gas exerts pressure on its container because the gas molecules constantly colliding with the walls of the container.
4. The average pressure of the atmosphere at sea level is 101 kilopascals or 760 mm of Hg under normal conditions.
5. The pressure necessary to support a column of mercury 760 mm is 101 kilopascals. Therefore 7.50 mm = 1 kPa.
6. A manometer is an instrument used to measure gas pressure.
7. Kinetic energy is the energy an object possesses because of its motion It is related to the mass and the velocity of the object.
8. Temperature is a measure of the average kinetic energy of the molecules of a substance.
9. The kelvin temperature scale is based on absolute zero. It has the unit as the Celsius scale degree, but a zero point at absolute (K = oC + 273o).
10. Heat energy flows from an object of higher temperature to one oflower temperature until both reach the same temperature.
11. The particles of a gas travel at random in a straight-line manner.
12. The particles of a solid appear to be vibrating about a point which is fixed with respect to neighboring particles.
13. The particles of a liquid appear to be vibrating about a point which is moving with respect to neighboring particles.
14. A plasma consists of electrons and positive ions in random motion. It is strongly affected by electric and magnetic fields.
More on Kinetic Theory:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page