Atomic Structure
![]()
Atomic Structure
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
ATOMlC STRUCTURE
The first seven chapters have been devoted to learning the language of chemistry. We will now look at the structure and properties of matter.
Much of the world depends on electricity. Most of our electricity travels from place to place along wires made of the element copper. Let us take a closer look at some copper. Suppose that we take a piece of copper wire and cut it into very small pieces. Would these pieces still be copper? How many times could we continue to divide a piece of copper and still have particles of copper? What do we have when we have divided the copper into smallest possible pieces?
We found earlier that the smallest piece of matter which would still be copper is called an atom. This atom, in turn, is made of smaller particles such as electrons, protons, and neutrons. Why do we believe that such small particles actually exist? What evidence do we have that they exist? Our modern concept of the atom is a result of generations of work. Even today, our knowledge of the atom is not yet complete.
We will devote this chapter to the study of atoms and their parts. In learning about the structure of the atom we will gain a better understanding of the properties of atoms taking chemical reactions.
8: 1 EARLY ATOMlC THEORY
Early thoughts concerning atoms were proposed by the philosopher, Democritus, about 400 B.C. He suggested that world was made of two things-- empty space and tiny particles which he called "atoms."
This word comes from the Greek atomos, meaning indivisible. Thus, he thought of atoms as smallest possible particles of matter. He also thought there different types of atoms for each material in the world. His was very general and was not supported by experimental evidence.
The belief in the existence of atoms was not accepted centuries because it contradicted the teachings of Aristotle who believed that matter was continuous and made of one substance called hyle.
Aristotle's teachings were accepted until the 17th century. Then, many people began to have doubts and objections to his theory.
Isaac Newton and Robert Boyle published articles on their belief in the atomic nature of elements. Their works offered no proof, they were explanations of the known, with no predictions of the unknown. It was up to an English chemist, John Dalton to offer a logical hypothesis about the existence of atoms.
8:2 LAW OF CONSERVATlON OF MASS
During the early 1800's, Dalton studied certain observations made by others concerning chemical changes. Antoine Lavoisier, a Frenchman, had made one of these discoveries. He found that when a chemical change occurred in a closed system, the mass the reactants before and a chemical change equals the mass of the products before the change.
In all tests of reactions in a closed system, he found that mass remains constant. He proposed that, in ordinary chemical reactions matter can be changed in many ways but it cannot be created nor destroyed. Today this law is called the Law of Conservation of Mass.

8:3 LAW OF DEFlNlTE PROPORTlONS
The work of another French chemist, Joseph Proust, also came to the attention of Dalton. Proust had observed that specific substances always contain elements in the same ratio by mass. For example, table salt is made of sodium and chlorine. The ratio of the mass of sodium to the mass of chlorine in any sample of pure salt is always the same.
No matter where the sample is obtained, how it is obtained, or how large it is, the ratio of the mass of sodium to the mass of chlorine is always the same. This principle is known as the Law of Definite Proportions.
8:4 DALTON'S HYPOTHESlS
Dalton was trying to explain the findings of Lavoisier and Proust when he formed the basis of our present atomic theory. He stated that all matter is composed of very small particles called atoms, and that these atoms could not be broken apart. Dalton's ideas were like those of Democritus. However, Dalton believed that atoms were simpler than particles of air or rock, and that atoms of different elements were quite unlike but each element was made of atoms that were exactly alike. He stated that atoms can unite with other atoms in simple ratios to form compounds.
These last two sentences are the key to the atomic theory. We can see how Dalton's ideas explain the two laws of Lavoisier and Proust. If atoms could not be destroyed, then they must simply be rearranged in a chemical change.
The total number and kind of atoms must remain the same. Therefore, the mass before a reaction must equal the mass after a reaction. If the atoms of an element are always alike, then all atoms of a particular element must have the same mass.
According to Dalton, all sodium atoms have the same mass and all chlorine atoms have the same mass. When a sodium atom combines with a chlorine atom, salt is formed.
The same is true of any pair of these atoms. Therefore, the ratio of the mass of sodium to the mass of chlorine must be the same for any sample of salt. He believed this reasoning would hold true for any given material.
Experiments have shown that Dalton's ideas are not entirely correct. As we will see later, not all atoms of the same element have exactly the same mass. However, by changing the word ''mass'' to ''average mass," we can use Dalton's ideas today.
8:5 LAW OF MULTlPLE PROPORTlONS
Dalton stated a second law based on his own atomic theory but not based on experimental data. The ratio of masses of one element which combines with a constant mass of another element can be expressed in small whole numbers. This statement is called the law of multiple proportions. Do you see why these numbers cannot be fractions? Atoms cannot be divided. A fraction of an atom does not exist.
Dalton's atomic theory:
1. All matter is composed of atoms.
2. All atoms of the same element are identical.
3. Atoms of different elements are different.
4. Atoms unite in definite ratios to form compounds.
At about the same time that Dalton formed his atomic theory, J. L. Gay-Lussac, a French chemist, made an interesting observation. He was working with gas reactions at constant temperature and pressure. He noted that, under constant conditions, the volumes of reacting gases and gaseous products were in the ratio of small whole numbers.
A few years later, Amadeo Avogadro, an Italian physicist, explained Gay-Lussac's work using Dalton's theory. Avogadro's hypothesis also concerned gases at the same temperature and pressure. He stated that equal volumes of gases, under the same conditions, had the same number of molecules. These observations sped the acceptance of Dalton's theory.
The atomic theory and the law of multiple proportions, as stated by Dalton, have been tested and are accepted as correct. There are however major exceptions to some of Dalton's statements. These differences will be considered as we form our modern model of the atom in the sections that follow.
8:6 PARTS OF THE ATOM
Experiments by several scientists in the middle of the 19th century led to the conclusion that the atom consisted of smaller particles. Using a tube such as the one in Figure 8-3, it was possible to learn a great deal about atoms. In each end of the tube, there was a metal piece called an electrode. The positive terminal was called the anode. The negative terminal was called the cathode. Careful observation revealed rays in the tube. Since these rays appeared to begin at the cathode and travel toward the anode, they were called cathode rays.
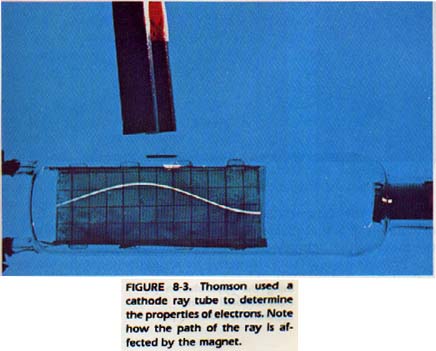
In 1897, J. J. Thomson, an English scientist, did some skillful research on the cathode rays. As a result, Thomson is generally credited with the discovery that the rays consisted of electrons. Thomson built a cathode ray tube to subject the rays to both a magnetic field and an electric field.
He measured the bending of the path of the cathode rays and was then able to determine the ratio of the electron's charge to its mass, Robert Millikan, an American, obtained the first accurate measurement of an electron's charge. He used a device like the one in Figure 8-4.
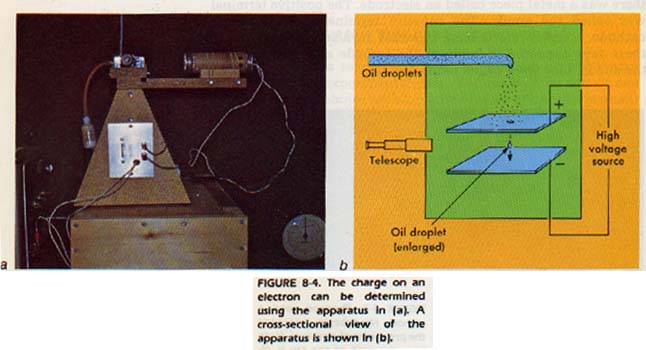
Electrons were transferred from the brass atomizer to oil droplets. These negatively charged droplets, under the influence of gravity, fell through a vacuum chamber. The charge on the plates was adjusted to just offset the gravitational force on the droplet.
Millikan could calculate the charge on the droplet. He found that the charges on the droplets varied. However, each charge was a multiple of one small charge. He concluded (correctly) that this small charge must be the charge on a single electron. This charge is now the standard unit of negative charge. The electron and charge on the electron may be represented by the symbol e-
Using the data of Thomson and Millikan, it was possible to calculate the actual mass of the electron. Its mass was only 1/1837 the mass of the lightest atom known, the hydrogen atom.
Protons were also discovered in an experiment involving a cathode ray tube modified such as the one in Figure 8-5.
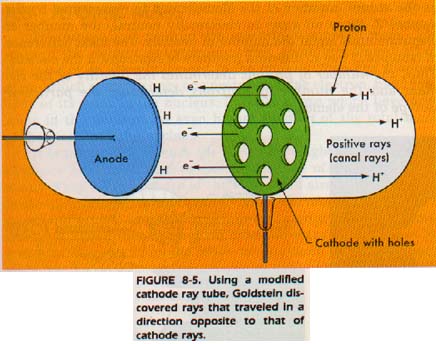
Rays were discovered traveling in the direction opposite to that traveled by the cathode rays. Later it was shown that these rays possessed a positive charge and J. J. Thomson showed they consisted of particles.
The particles he found have the same amount of electric charge as an electron. However, the charge is opposite in sign to that on the electron. These particles are now called protons. The atoms of hydrogen gas he used in the tube consist of a single proton and a single electron. Thomson calculated that the mass of the proton was just about 1836 times that of the electron. The proton is now the standard unit of positive charge (1+).
A third particle remained unobserved for a long time. However, its existence had been predicted by Lord Rutherford, an English physicist, in 1920. The first evidence of the particle was obtained by Walter Bothe in 1930.
Another English scientist, James Chadwick, repeated Bothe's work in 1932. He found high energy particles with no charge and with essentially the same mass as the proton. These particles are now known as neutrons. We now know of a number of other subatomic particles. Scientists have predicted the existence of others. Subatomic particles will be studied in greater detail in Chapter 28.
Dalton had assumed that atoms could not be broken into smaller particles. The discovery of subatomic particles led to a major revision of Dalton's atomic theory to include this new information.
8:7 lSOTOPES AND ATOMlC NUMBER
While working with neon, J. J. Thomson observed what seemed to be two kinds of neon atoms. They were exactly alike chemically, but slightly different in mass.
Isotope is used to describe different atoms of the same element. Isotopes possess the same number of protons but a different number of neutrons. Another English scientist, Henry Moseley found the wavelength of X-rays produced in an X-ray tube was characteristic of the metal used as the anode. The wavelength depended on the number of protons in the nucleus of the atom and was always the same for a given element.
This number of protons is known as the atomic number of the element and is represented by symbol Z.
Since an atom is electrically neutral, the number of electrons must equal the number of protons. The mass difference of isotopes is due to different numbers of neutrons in the nucleus. Thus, the number of protons determines the identity of the element and the number of neutrons determines the particular isotope of the element.
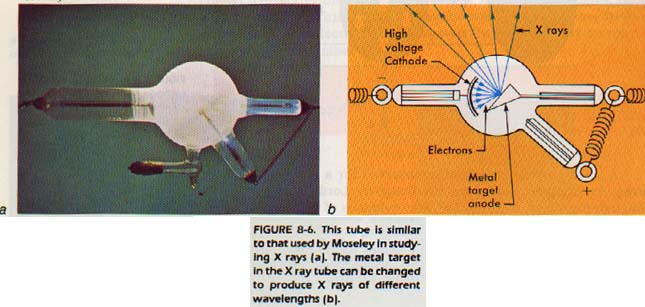
The particles which make up the nucleus (protons and neutrons) are called nucleons.
The total number of nucleons in an atom is called the mass number of that atom. The symbol for the mass number is A. A particular kind of atom containing a definite number of protons and neutrons is called a nuclide. The number of neutrons for any nuclide may be found by using the following.
number of neutrons = A - Z

8:8 ATOMlC MASS
The proton and neutron are essentially equal in mass. The mass of the electron is extremely small, therefore, almost all of the mass of an atom is located in the nucleus. Even the simplest atom, which contains only one proton and one electron, has 1836/1837 of its mass in the nucleus. In other atoms which have neutrons in the nucleus, an even higher fraction of the total mass of the atom is in the nucleus.
It is possible to discuss the mass of a single atom. However, chemists have continued to use the masses of large groups of atoms. They do so because of the very small size of the particles in the atom.
Chemists have chosen one mole, which is Avogadro's number of atoms, as a standard unit for large numbers of atoms. Chemists chose this number so that the mass of NA atoms in grams is equivalent to the mass of one atom in atomic mass units.
We know that the unit gram was defined as 1/1000 the mass of the International Standard Kilogram.
What is an atomic mass unit? To measure atomic masses, an atom of one element was chosen as a standard, and the other elements were compared with it.
Scientists used a carbon nuclide, carbon-12, as the standard for the atomic mass scale. The carbon-12 atom is the nuclide of carbon with 6 protons and 6 neutrons in the nucleus. One carbon-12 atom is defined as having a mass of 12 atomic mass units. An atomic mass unit is defined to be 1/12 the mass of the carbon-12 nuclide.
The particles which make atoms are called subatomic particles. The subatomic particles which we have studied thus far have the following masses:
electron = 9.11 x 10-28 g = 0.000549 amu (g/mol)
proton = 1.673 x 10-24 g = 1.0073 amu (g/mol)
neutron = 1.675 x 10-24 g = 1.0087 amu (g/mol)
If it were possible to take exactly 12 grams of carbon-12 atoms and count them, we would have Avogadro's number of atoms.
Look carefully at Table 8-3, Atomic Masses. Notice that many of the elements have a mass in atomic mass units which is close to the total number of protons and neutrons in their nuclei.
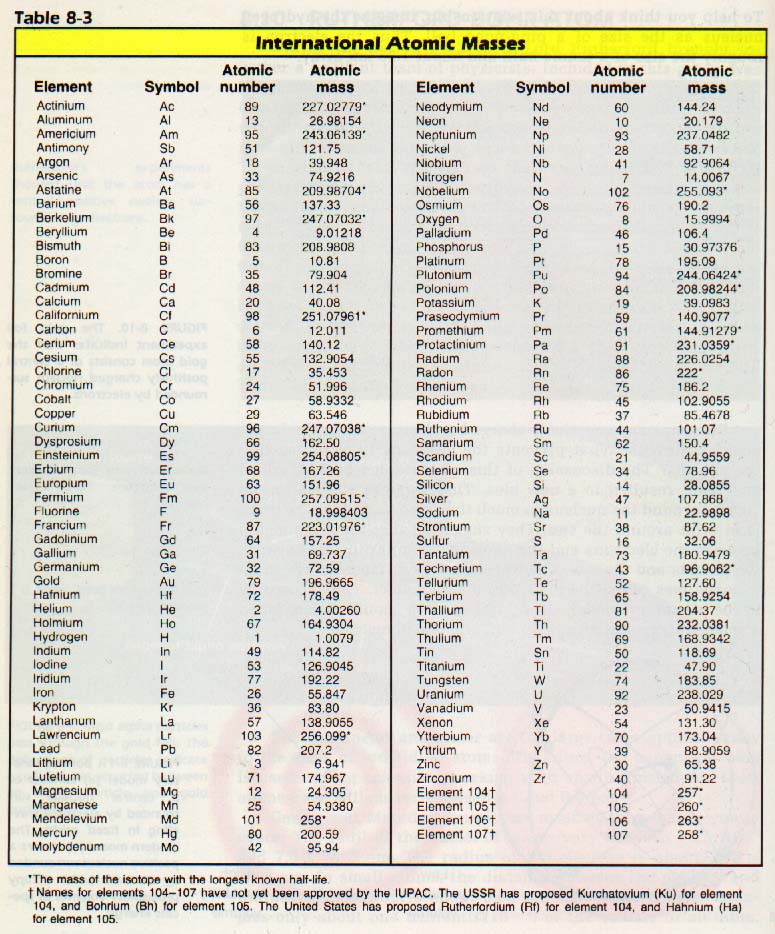
However, some do not. What causes the mass of chlorine or copper, for example, to be about halfway between whole numbers? The numbers in the table are based on the "average atom" of an element. Most elements have many isotopic forms which occur naturally. It is difficult and expensive to obtain a large amount of a single nuclide of an element. Thus, for most calculations, the average atomic mass of the element is used.
8:9 AVERAGE ATOMlC MASS
Using a standard nuclide, there are two ways of determining masses for atoms of other elements. One method is by reacting the standard element with the element to be determined.
Using accurate masses of the two elements and a known mole ratio, the atomic mass of the second element may be calculated as a mass-mass problem.
Greater accuracy can be obtained by using a physical method of measurement in a device called a mass spectrometer. Its development was based on the early tubes of J. J. Thomson.
It is similar in design to the tube used by Thomson to find the charge/mass ratio of the electron. Using a mass spectrometer, we can determine the relative amounts and masses of the nuclides for all isotopes of an element.
The element sample, which is in the form of a gas, enters a chamber where it is ionized by hitting it with electrons. These ions are then propelled by electric and magnetic fields. As in the Thomson tube, the fields bend the path of the charged particles as shown in Figure 8-7.
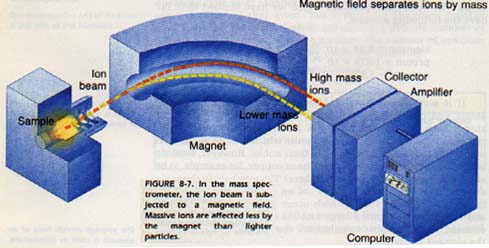
The paths of the heavy particles are bent slightly as they pass through the fields. The paths of the lighter particles are curved more. Thus, the paths of the particles are separated by relative mass.
The particles are caught and recorded electronically. One drawback of the instrument is that the ionization chamber, field tube, and detection device must all be in a vacuum. This vacuum must be equal to about one hundred-millionth (1/100 000 000) of normal atmospheric pressure.
8:9 AVERAGE ATOMIC MASS
Since the strength of the fields, and the speed and paths of the particles are known, the mass of the particles can he calculated. Once the masses of the isotopes and their relative amounts have been found, the average atomic mass can be calculated.
For example, it has been found that neon has two isotopes. They have masses of 19.992 and 21.991 amu. They are present in every neon sample in the ratio of 9: 1. In a sample of ten atoms, nine will be neon-20 and one will be neon-22. The average mass can then be found by this method:
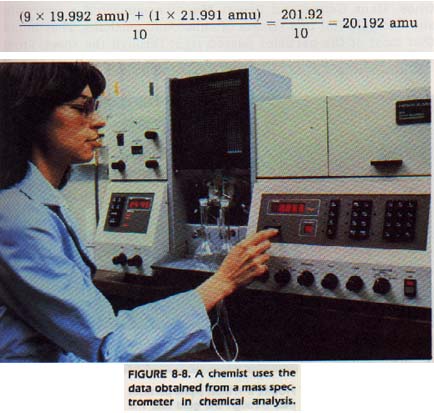
This average mass is called the atomic mass of an element. It is represented by the symbol M. The mass spectrometer has other uses. Geologists, biologists, petroleum chemists, and many other research workers use the mass spectrometer as an analytical tool.
8: 10 BUTHERFORD-BOHR ATOM
During the period 1912-1913, Lord Rutherford brought together a brilliant team of physicists. Included in this group was Niels Bohr, a young Dane. The beginnings of our modern concept of atomic structure were developed by this group through experiment and hypothesis.
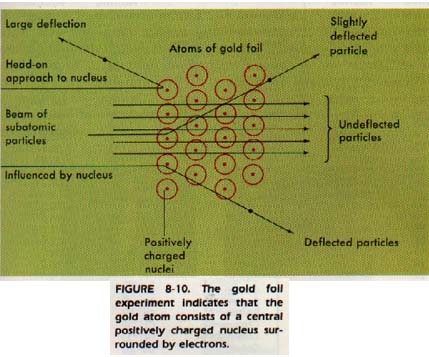
Experiments designed to reveal the structure of atoms were performed under Rutherford's direction. These experiments showed that the atom consists of a central, positively charged nucleus surrounded in some manner by electrons.
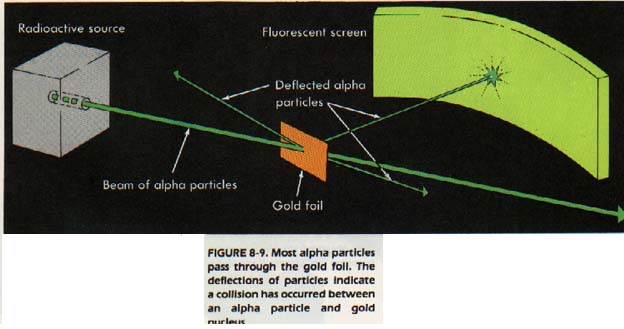
Hans Geiger and Ernest Marsden subjected a very thin sheet of gold foil to a stream of subatomic particles. They found that most of the particles passed right through the sheet.
From this observation Rutherford concluded that the atom is mostly empty space. They also found a few particles (about 1 in 8000) bounced back in almost the opposite direction from which they started.
Rutherford explained this observation as meaning that there was a very small "core" to the atom. The "core" contained all the positive charge and almost all the mass of the atom. This "core" is now called the nucleus.
The centimeter and meter are too large to be applied easily to the sizes of individual atoms. Therefore, the manometer (nm) is used among scientists working with small dimensions. Most atoms have a diameter between 0.1 and 0.5 nm.
Geiger and Marsden found that most of the atom is empty space. The radii of the nuclei of atoms vary between l.2 x 10-6 and 7.5 x lO-6 nm. The radius of the electron is about 2.82 x lO-6 nm. In small atoms, the distance between the nucleus and the nearest electron is about 0.05 nm. Thus, the nucleus occupies only about one trillionth (10-12) of the volume of an atom.
To help you think about this relationship, imagine the hydrogen nucleus as the size of a ping-pong ball. Thus the electron is roughly the size of a tennis ball, and about 1.35 km away!
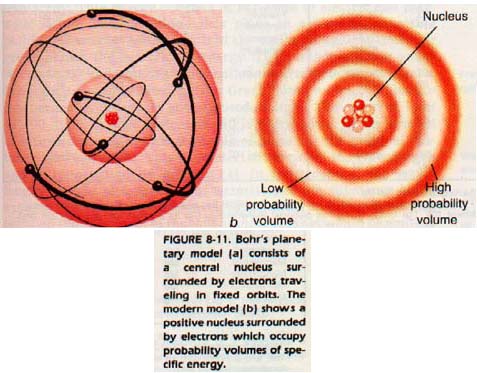
Electrons are negatively charged and attracted to the larger positive nucleus. What prevents the electrons from falling into the nucleus? The discussion of this question led by Rutherford and Bohr, resulted in a new idea.
They thought of electrons in "orbit" around the nucleus in much the same manner as the earth is in orbit around the sun. They suggested that the relationship between the electrons and the nucleus is similar to that between the planets and the sun.
The Rutherford-Bohr model of the atom is sometimes called the planetary atomic model. Thus according to the planetary model, the hydrogen atom should be similar to a solar system consisting of a sun and one planet.
8: 11 SPECTROSCOPY
When a substance is exposed to a certain intensity of light, heat, or some other form of energy, the atoms absorb some of the energy. Such atoms are said to be excited.
When atoms and molecules are in an excited state, energy changes occur. Each excited atom or molecule produces unique energy changes which can be used to identify it.
Radiant energy of several different types can be emitted (given off) or absorbed (taken up) by excited atoms and molecules. The methods of studying substances that are exposed to some sort of continuous exciting energy are called spectroscopy.
Light is one form of radiant, or electromagnetic energy. Other forms of electromagnetic energy are radio, infrared, ultraviolet, and X ray. This energy consists of variation in electric and magnetic fields. The variation takes place in a regular, repeating fashion.
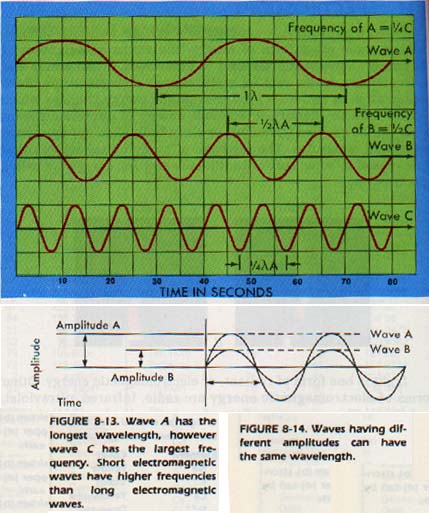
If we plot the strength of the variation against time, our graph shows the "waves" of energy. The number of wave peaks which occur in a unit of time is called the frequency of the wave.
Frequency is represented by the Greek letter nu, ν). All electromagnetic energy travels at the speed of light. The speed of light is 3.00 x 108 m/s in a vacuum and is represented by the symbol c.
A third important characteristic of waves is the physical distance between peaks. This characteristic is called the wavelength and is represented by lambda, λ.
In spectroscopy, the wavelength of light absorbed is characteristic of the substance being excited. The unique set of wavelengths absorbed by a substance is called the spectrum of that substance. This set would be emitted by all excited particles of the same substance.

We will work with absorption spectra, although emission spectra are also useful. Another wave property that is of importance is the amplitude of a wave, or its maximum displacement from zero. In Figure 8-l4, two waves are plotted on the same axes. Note that the amplitude of wave A is twice that of wave B, even though they have the same wavelength.
8: 12 VlSlBLE AND ULTRAVlOLET SPECTROSCOPY
Absorption and emission spectra are the fingerprints of the elements.
Each element has its own unique set of wavelengths that it absorbs or emits.
Electromagnetic energy with a wavelength between 700 and 400 manometers lies in the visible spectrum. This small band of visible radiation has given chemists and physicists much information about the elements. Some elements (rubidium, cesium, helium, and hafnium) were actually discovered through its use.
The visible spectrum may also be used for finding the concentration of substances, for analyzing mixtures, and for analyzing complex ions. Almost any change involving color can be measured using visible spectroscopy,
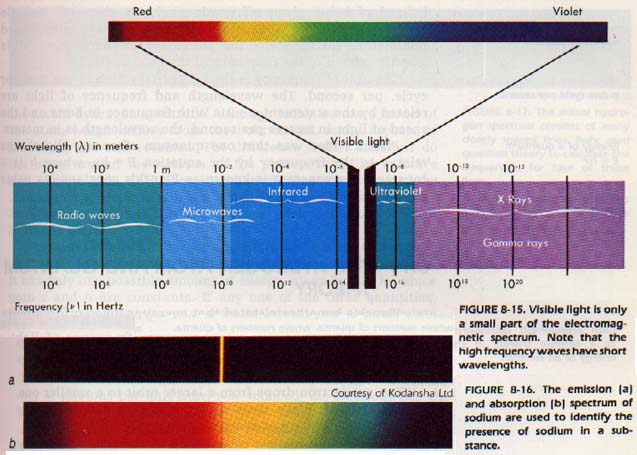
Ultraviolet radiation (400-200 nm) can also be used to study atomic and molecular structure. Both ultraviolet spectra and visible spectra are produced by electron changes.
The ultraviolet spectrum of an element or compound consists of bands rather than lines. Ultraviolet radiation has such high energy, it violently excites the electrons. The transition of the electrons from the ground state to such highly excited states causes changes in the molecule being studied.
Bonds between atoms may even be broken. Visible radiations are not as destructive because they have less energy than UV radiation. Ultraviolet spectroscopy is used for the same types of analyses as visible spectroscopy.
In order to describe completely the electronic structure of a substance, both types of analysis must be used.
8: 13 PLANCK'S HYPOTHESlS
Once Bohr had developed the basic outline of his planetary model of the atom, he used the quantum theory which had been stated by a German, Max Planck. Planck assumed that energy, instead of being given off continuously, is given off in packets, or quanta.
Quanta of radiant energy are often photons. He further stated that the amount of energy given off is directly related to the frequency of the light emitted.
The unit of frequency is the hertz (Hz) which is one peak, cycle, per second.
The wavelength and frequency of light related by the statement c = f λ . With frequency in hertz and speed of light in meters per second, the wavelength in meters. The idea was that one quantum of energy (light) related to the frequency by the equation E = hν, where h is constant. The constant is known as Planck's constant. Its is 6.63 x 10-34joules per hertz (cycles/second).
8: 14 THE HYDROGEN ATOM AND QUANTUM THEORY
Planck's hypothesis stated that energy is given off in quanta instead of continuously.
Bohr pointed out that the absorption of light by hydrogen at definite wavelengths means definite changes in the energy of the electron. He reasoned that the orbits of the electrons surrounding a nucleus must have a definite diameter.
According to Bohr, electrons could occupy only certain orbits. The only orbits allowed were those whose differences in energy equaled the energy absorbed when the atom was excited.
Bohr thought that the electrons in an atom could absorb or emit energy only in whole numbers of photons. In other words, an electron could emit energy in one quantum or two quanta, but not in fractions of quanta.
Bohr pictured the hydrogen atom as an electron circling a nucleus at a distance of about 0.053 nm. He also imagined that this electron could absorb a quantum of energy and move to a larger orbit.
Since a quantum represents a certain amount of energy, the next orbit must be some finite distance away from the first. If still more energy is added to the electron, it moves into a still larger orbit, and so on.
When an electron drops from a larger orbit to a smaller one, energy is emitted. Since these orbits represent definite energy levels, a definite amount of energy is radiated.
The size of the smallest orbit which an electron can occupy, the one closest to the nucleus, can be calculated. This smallest orbit is called the ground state of the electron. Bohr calculated the ground state of the hydrogen electron. Using quantum theory, he calculated the frequencies for the lines that should appear in the hydrogen spectrum, His results agreed almost perfectly with the actual hydrogen spectrum.
Although today we use a model of the atom which differs from the Bohr model, many aspects of his theory are still retained. The difference is that electrons do not move around the nucleus as the planets orbit the sun.
We will explore this difference in the next chapter However, the idea of energy levels is still the basis of atomic theory. The energy level values calculated by Bohr for the hydrogen atom are still basically correct.
We can restate the discussion as follows: A wave of a certain frequency has only one possible wavelength, given by h = c/ν. It has only one possible amount of energy, given by E = hν, since both c and h are constants. If any one of the three quantities, frequency, wavelength, or energy, is known, we can calculate the other two.
The visible and ultraviolet spectra produced by a compound can be used to determine the elements in the compound. Each line in a spectrum represents one frequency of light.
Because the velocity of light is always constant, each frequency means a certain energy. This energy is determined by the movement of electrons between energy levels which are specific for each element. The same set of energy levels will always produce the same spectrum.
8: 15 PHOTOELECTRIC EFFECT
Before leaving quantum theory, we will consider another observation explained by this theory. Recall that quanta of light energy are referred to as photons.
It had been known for some time that light falling on the surface of certain substances would cause electrons to be emitted. There was, however, a puzzling fact about this change.
When the intensity of light (the number of photons per unit time) was reduced, the electrons had the same energy. However, there were fewer electrons emitted. Albert Einstein pointed out that Planck's hypothesis explained this observation.
A certain amount of energy is needed to remove an electron from the surface of a substance. If a photon of greater energy strikes the electron, the electron will move away from the surface. Since it is in motion, the electron has some kinetic energy. Some of the energy of the photon is used to free the electron from the surface. The remainder of the energy becomes the kinetic of the electron.
If light of one frequency is used, then the escaping from the surface of the substance will all have the energy.
This emission of electrons is called the photoelectric effect. If the light intensity is increased, but the frequency the same, the number of electrons being emitted will increase.
If the frequency of the light is increased, the energy of the photon is increased. The amount of energy that must be used to free the electron from the atom is constant for a given substance.
The electrons now leave the surface with a higher kinetic energy than they did with the other frequency.
Planck's hypothesis, together with Einstein's explanation, confirmed the particle nature of light.

SUMMARY
1. Democritus proposed the earliest recorded atomic theory.
2. Modern atomic theory dates from John Dalton's hypothesis. He use of the law of conservation of mass and the law of definite proportions to state that all matter is formed of indivisible particles called atoms. All atoms of one element are the same. Atoms of different elements are unlike, and atoms can unite with one another in simple whole number ratios
3. Modern atomic theory differs foam Dalton's atomic theory due to discovery of subatomic particles and isotopes.
4. An electron is a negatively charged particle with a very small mass. A proton is a positively charged particle with a mass 1836 times the of an electron. A neutron is an uncharged particle with a mass about same as the mass of a proton.
5. All atoms of an element contain the same number of protons in nuclei.
6. Atoms containing the same number of protons but different numbers of neutrons are isotopes of the same element.
7. The atomic number (Z) of an element is the number of protons in its nucleus. The mass number (A) of an atom is the number of particles in its nucleus.
8• The atomic mass of an element is the weighted average mass, (M) of all the natural isotopes of the element. It is the mass of an average individual atom of the element compared with 1/12 the mass of the carbon-12 atom.
9. Atomic mass can be measured with the mass spectrometer.
10. Atoms are extremely small and consist mostly of space.
11. Rutherford and Bohr pictured the atom as coexisting of a central nucleus surrounded by electrons in orbits.
l2. Substances excited by an energy source emit light in definite wavelengths called a spectrum.
l3. Visible and ultraviolet spectroscopy are used to study the wavelengths of light absorbed and emitted by electrons in atoms.
14. planck stated that energy is radiated in discrete units called quanta. A photon is a quantum of light energy.
l5. The energy of a quantum of radiation varies directly as the frequency of the radiation (E = hν).
16. Quantum theory helped Bohr explain the hydrogen spectrum, and thus, to calculate the orbits for the hydrogen atom.
l7. The photoelectric effect is the loss of electrons from a substance, caused by photons striking its surface.
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page