Liquids
![]()
Liquids
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
LIQUIDS
According to the kinetic theory, if the temperature of a solid is raised, the velocity of the particles should increase. As the increases, the particles collide with each other with greater force (Bam, Bam, Biff, Biff). Thus, they are forced farther apart.
Almost all solids and liquids expand when they are heated because of this in velocity. If the temperature of a solid is raised sufficiently, the particles will move far enough apart to slip over one another.
The ordered arrangement of the solid state breaks down. When such a change takes place, we say the solid has melted.
If particles are in the liquid state, there will be a certain temperature (and pressure) at which the particles travel so slowly that they can no longer slip past one another.
All pure liquids have a definite freezing point and all pure solids have a definite melting point. For a substance, the freezing point of the liquid form is the same temperature as the melting point of the solid.
17:1 VAPOR EQUlLlBRlUM
The average kinetic energy of atoms or molecules in a gas is a constant for all substances at a given temperature. This average kinetic energy can be calculated for any particular temperature.
If we were to measure the kinetic energy of individual atoms or molecules in a gas, we would find that few had the predicted kinetic energy. Some molecules would have more and some would have less kinetic energy than the average. Most would have a kinetic energy close to the calculated amount. However, we would sometimes find a molecule with a kinetic energy considerably above or below the average.
All that has been said about the collisions of particles in a gas is also true of particles in a solid or a liquid. A molecule in a liquid, because of several rapid collisions with other molecules, might gain kinetic energy considerably above the average value.
Imagine that molecule on the surface of the liquid. If it has enough kinetic energy to overcome the attractive force of nearby molecules, it may escape from the liquid surface.
The same process may also occur at the surface of a solid. The molecules which escape from the surface of a solid or a liquid form a vapor. This vapor is made of molecules or atoms of the substance in the gaseous state. A gas and a vapor are the same. We usually use the word gas for those substances which are gaseous at room temperature. Vapor is used for the gaseous state of substances which are liquids or solids at room temperature.
A molecule of a solid or liquid which has escaped the surface behaves as a gaseous molecule. It is possible for this molecule to collide with the surface of the liquid it left. If its kinetic energy is sufficiently low at the time of such a collision, the molecule may be captured and again be a part of the liquid. However, in an open container, there is little chance of the molecule returning to the surface it left.
If the solid or liquid is in a closed container, then there is an increased chance of the molecule returning to the surface. In fact, a point will be reached where just as many molecules return to the surface as leave the surface. There will be a constant number of molecules in the solid or liquid phase, and a constant number of molecules in the vapor phase.
Such a situation is known as an equilibrium condition. It is a special kind of equilibrium, called dynamic equilibrium. It is called dynamic because molecules are continuously escaping from and returning to the surface. However, the overall result remains constant.
When a substance is in equilibrium with its vapor, the gaseous phase of the system is said to be saturated with the vapor of that substance.

17:2 THE PRlNClPLE OF LE CHATELIER
The vapor phase exerts a pressure which is dependent on the temperature. The higher the temperature, the higher the vapor pressure. A liquid and its vapor will reach equilibrium at a specific pressure for any particular temperature. This shifting of the Equilibrium was observed and described by the Frenchman le Chatelier in 1884.
Le Chatelier's principle is expressed as: If stress is applied to a system at equilibrium, the system readjusts so that the stress is reduced. The stress may be a change in temperature, pressure, concentration, or other external force.
For example, an ice skater can skim over the ice with little effort. The skate blades are not really touching the ice but are traveling on a thin film of liquid water. The presence of this water can be explained by Le Chatelier's principle.
Both pressure and temperature must be considered in this example. We will discuss pressure first. The entire weight of the skater is directed onto the ice through the blades on the skates. The surface area of the blades in contact with the ice is probably less than 15 cm2. Thus, the blades exert a great pressure on the ice at the points of contact.
A piece of ice (solid) occupies a greater volume than an equal mass of water (liquid). Therefore, the change of ice to water will tend to reduce the pressure (stress) caused by the blades. Increased pressure causes a reduction in volume. As a result the blades travel on a film of liquid water.
Temperature also plays an important role in skating. Friction between the ice and the blades of the skates warms the blades. This increased temperature (stress) will be relieved by the now of heat into the ice. Increased temperature causes an increase in molecular motion. The low energy ice molecules are changed into more energetic water molecules. The blades will be cooled by this change. Both the increased pressure and the increased temperature enable the skater to glide over the ice with very little friction.

17:3 MEASURING VAPOR PRESSURE
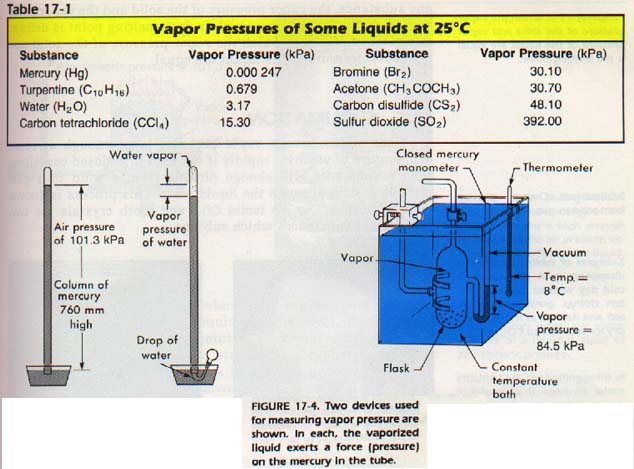
Many techniques are available to measure vapor pressure. Figure 17-4 shows two methods of finding the vapor pressures of substances. The apparatus in Figure 17-4 is especially useful for finding the vapor pressure of solids at elevated temperatures. Table 17-1 gives the vapor pressures of some substances near room temperature. Substances with low vapor pressure have strong intermolecular forces. Those with high vapor pressures have weak intermolecular forces.
17:4 MELTlNG POlNT
Consider the phenomenon of melting in a closed container. In a mixture of solid and liquid states, there will be a dynamic equilibrium between the molecules of the solid and liquid. Remember, though, that each state is also in equilibrium with its vapor. Since there is only one vapor, the solid and liquid have the same vapor pressure.
The melting point is defined as the temperature at which the vapor pressure of the solid and the vapor pressure of the liquid are equal.
17:5 SUBLlMATlON
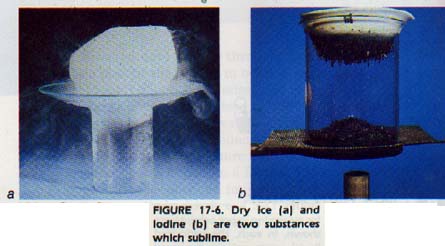
Some solids have a vapor pressure large enough at room temperature to vaporize rapidly if not kept in a closed container. Such a substance will change directly from a solid to a gas, without passing through the liquid state. This process is known as sublimation. Dry ice (solid CO2 and moth crystals are two examples of substances which sublime.
17:6 BOlLlNG POlNT
A liquid and its vapor can be at equilibrium only in a closed container. The molecules leaving the surface of a liquid have little chance of returning if the liquid container is open to the air.
When a liquid is exposed to the air, it may gradually disappear. The disappearance is due to the constant escape of molecules from its surface. The liquid is said to evaporate.
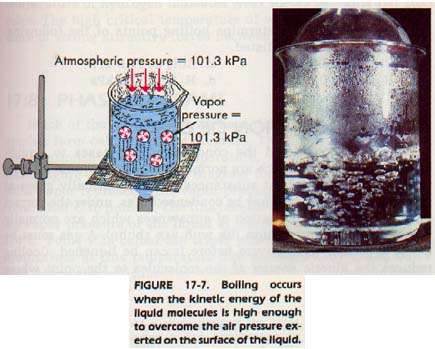
As the temperature of a liquid is increased, the vapor pressure of that liquid increases because the kinetic energy of the molecules increases. Eventually, the kinetic energy of the molecules becomes large enough to overcome the internal pressure of the liquid.
The internal pressure is due to the air pressing on the liquid surface. When this pressure is overcome, the molecules are colliding violently enough to push each other apart. They are pushed far enough apart, in fact, to form bubbles of gas within the body of the liquid. These bubbles rise to the surface of the liquid because the vapor of which they are composed is less dense than the surrounding liquid.
At this point, the liquid is boiling. The normal boiling point is the temperature at which the vapor pressure is equal to standard atmospheric pressure, 101 kPa.
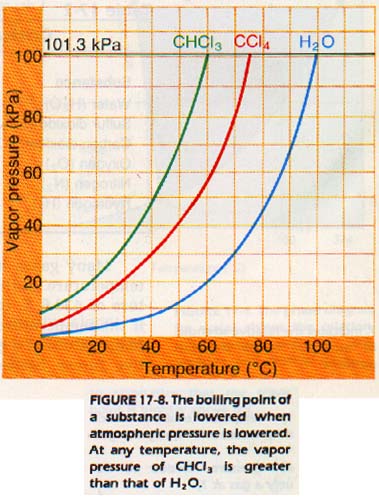
Boiling point is a function of pressure. At lower pressure, the boiling point is lower.
Note carefully the difference between evaporation and boiling. Evaporation occurs only at the surface. Boiling, on the other takes place throughout the body of a liquid.
Adding heat to a liquid at its boiling point will cause it to change to a gas rapidly by boiling. In a like manner, if we remove heat from a gas at the boiling point of that substance the gas will change to a liquid. Boiling point of a liquid is also the condensation point of the vapor state of the liquid.
Different liquids boil at different temperatures A liquid which boils at a low temperature and evaporates rapidly at room temperature is said to be volatile. Examples of volatile liquids are alcohol, gasoline, and ether.
Liquids which boil at high temperature and evaporate slowly at room temperature said to be nonvolatile. Two liquids are molasses and honey.
Volatile substances have high vapor pressures; nonvolatile substances have low vapor pressure sat room temperature.
17:7 LlQUEFACTION OF GASES
We have considered the condensation of gases to liquids only for substances which are normally solids or liquids at room temperature. What about substances that are normally gases at room temperature? Can they be condensed? Yes, under the correct conditions.
The condensation of substances which are normally gases is called liquefaction.
A gas must be below a certain temperature before it can be liquefied. Cooling reduces the kinetic energy of the molecules to the point where the van der Waals attraction is sufficient to bind the molecules together. It is also necessary to compress some gases. The van der Waals forces are effective for only short distances. Compression forces the molecules of these gases close enough for the van der Waals forces to take effect.
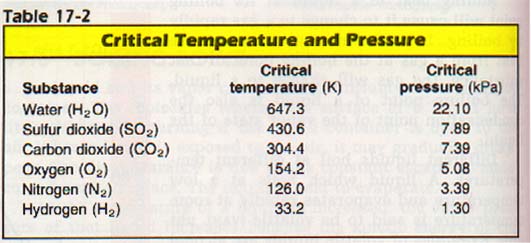
For every gas, there is a temperature above which no amount of pressure will result in liquefying the gas. This point is called the critical temperature, Tc , of the gas. The critical pressure, Pc , is the pressure that will cause the gas to liquefy at the critical temperature.
Many gases have critical temperatures above normal room temperature. Sulfur dioxide, for example, has a critical temperature of 430 K. It can be liquefied by increased pressure alone, if the temperature is not allowed to exceed 430 K. The critical temperature of a gas is an indication of the strength of the attractive forces between its atoms or molecules. The low critical temperature of hydrogen indicates weak forces between its molecules. The high critical temperature of water indicates the existence of strong attractive forces between molecules.
7:8 PHASE DlAGRAMS
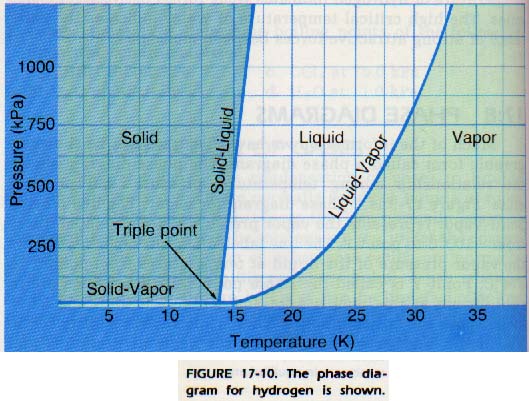
Much of the information we have discussed can be shown in form called a phase diagram. The phase diagram shows relationship among temperature, pressure, and physical state. Figure 17-9 is a phase diagram for water. The line labeled Solid -Vapor represents the vapor pressure of ice at temperatures from -100oC to point Y.
The line labeled Liquid-Vapor represents the vapor pressure of the liquid at temperatures from point Y to 374oC. Point Y is called the triple point. All three states are in equilibrium at this temperature and pressure, 0.OloC and 0.611 kPa.
Above the critical point, X, there is no vapor pressure curve. The liquid and gaseous states are the same at pressures and temperatures above this point.
Tb is the boiling point and Tm is the melting point. The melting point occurs where the Solid-Liquid equilibrium line is cut by the standard atmospheric pressure line.
It is important to realize that the vapor pressure of the liquid and solid (see point Tm in Figure 17-9) is not equal to atmospheric pressure at this point. The line YZ simply indicates the pressure temperature conditions under which the solid and liquid can be in equilibrium.
Only the solid-vapor and liquid-vapor lines represent vapor pressure information.
The boiling point is that temperature at which the liquid-vapor equilibrium curve is cut by the pressure line of 101 kPa (see dotted lines crossing at Tb in Figure 17-9).
Note that the solid-liquid equilibrium line for water has a negative slope. A negative slope indicates that a rise in pressure will lower the freezing point.
As was pointed out in Section 17:2 about the ice skater, water expands when it freezes. Such a change is unusual. Most substances contract when they freeze and their solid-liquid equilibrium line has a positive slope.
Figure 17-10 shows the phase diagram for hydrogen. Note the positive slope for the Solid-Liquid equilibrium line.
17:9 ENERGY AND CHANGE OF STATE
We have seen that the loss or gain of heat energy from a system has an effect upon the equilibrium which exists between states. It is important for the chemist to be able to treat these energy changes quantitatively in order to describe completely a change which has taken place in a system.
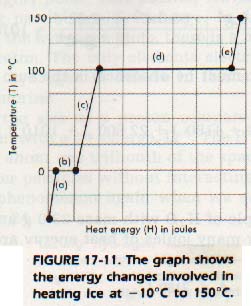
When heat is added to a solid substance, the temperature of the object increases until the melting point of the substance is reached. Upon the addition of more heat, the substance begins to melt. The temperature, however, remains the same until all of the substance has melted.
Before the melting point is reached, the added energy increases the kinetic energy of the molecules. In other words, the temperature is raised. At the actual melting point, the position of the particles is changed. In other words, the physical state was changed and the potential energy is increased.
The heat required to melt 1 g of a specific substance at its melting point is called the heat of melting, or heat of fusion, Hf , of that substance. A similar phenomenon takes place at the boiling point. The heat required to vaporize 1 g of a substance at its boiling point is called the heat of vaporization, Hv, of the substance.
17:10 HYDROGEN BONDlNG
In a number of substances, the predicted melting and boiling points differ from the observed ones. Remember that these changes of state can be predicted from a knowledge of atomic and molecular structure. It is the structure which affects interatomic and intermolecular forces.
Many of the substances which do not behave as predicted have two things in common. First, their molecules contain hydrogen, and second, the hydrogen is covalently bonded to a highly electronegative atom.
Under these conditions, the electronegative atom has almost complete possession of the electron pair shared with the hydrogen atom. The molecule is therefore highly polar. This polarity leaves the hydrogen atom with a strong partial positive charge. In fact, a the point of attachment of the hydrogen atom, there is a nearly bare hydrogen nucleus, or proton.
The only elements electronegative enough to cause bonded hydrogen to behave in this manner are nitrogen, oxygen, and fluorine.
Consider the size of a proton compared to the size of the next largest ion with a 1+ charge, Li+. The H+ ion has a full positive charge in about one trillionth of the space. It simply will not exist near other particles without interacting with them. We will discuss this phenomenon again when we study the hydronium ion in Chapter 24.
In a molecule containing hydrogen bonded to a highly electronegative element, the proton is not completely bare. However, the partial charge on the hydrogen end of the molecule is much more concentrated than that at the positive end of an average dipole.
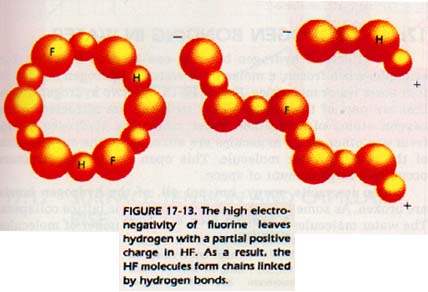
Hydrogen is the only element to exhibit this because all other positive ions have inner levels of shielding electrons in their nuclei. In a substance composed of polar molecules containing hydrogen, the hydrogen atom is attracted to the negative portion of other molecules.
Since the hydrogen atom has been reduced to a proton with almost no electrons, the attractive force is strong. However, it is not nearly as strong as a chemical bond. The attractive force in such substances is called the hydrogen bond.
The result of the hydrogen bond is that hydrogen atom tends to hold the two molecules firmly to each other.
Because of its special properties, the hydrogen bond has a greater effect than another dipole with the same electronegativiy difference.
Hydrogen bonding is really just a subdivision of large class of interactions called dipole attractions. However it is considered apart from other dipole attractions because it has a greater effect on the properties of substances.
17:11 HYDROGEN BONDlNG IN WATER
The effects of hydrogen bonding can be seen in water. For example, when frozen, a molecule of water is hydrogen bonded to four other water molecules, Figure 17-15.
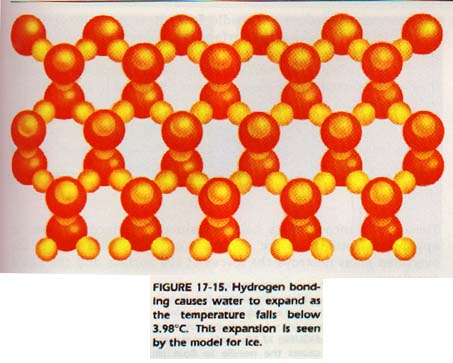
The two hydrogen atoms that are part of the central water molecule are attracted to oxygen atoms of two other water molecules. Hydrogen from two other water molecules are attracted to the oxygen of the central water molecule. This open crystalline structure occupies a large amount of space.
When ice melts, many, but not all, of the hydrogen bonds are broken. As some of the bonds are broken, the lattice collapses. The water molecules move closer. The same number of molecules occupy less space. Thus, water is more dense than ice. As water is heated above OoC, more hydrogen bonds are broken, and the molecules continue to move closer.
Finally, at 4oC, most af the hydrogen bonds have been broken. Above 4oC, the water expands with increased temperature. At this temperature, the density of water starts to decrease. Now , we can understand why water has its maximum density at 4oC.
17:12 SURFACE TENSlON AND CAPlLLARITY
Obtain a needle and a glass of water. With tweezers, place the needle carefully on the surface of the water. Be sure there is no soap on your hands, the tweezers, or the needle. With a little practice, you will be able to float the needle on the surface of the water. Why does the needle float? Have you ever poured a drink into a glass so that the surface of the liquid was higher than the rim of the glass?
The particles at the surface have special properties because they are subjected to unbalanced forces as shown in Figure 17-17.
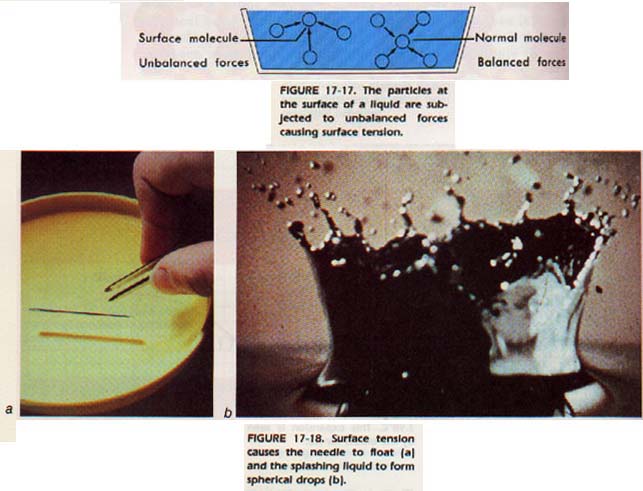
These unbalanced forces help explain the surface tension, or apparent elasticity of the surface. You know that jarring the overfilled glass destroys the forces at the surface and the liquid overflows.
The net force not only accounts for the surface tension, but also helps explain why liquids form spheres when dropped. The net force acting on a surface particle is directed perpendicularly into the liquid. Thus, the body of a liquid is pulling the surface molecules inward. Since a sphere has the least surface area for any given bulk, liquids tend to assume a spherical shape when dropped.
Quantitatively, surface tension is expressed as force per unit volume. The unbalanced force also accounts for the phenomenon known as capillary rise. If there is an attractive force between a liquid and the solid wall of the capillary tube, the liquid will rise in the tube.
Capillary rise for water is shown in Figure 17-19. The attractive force relieves the unbalanced force on the surface molecules.
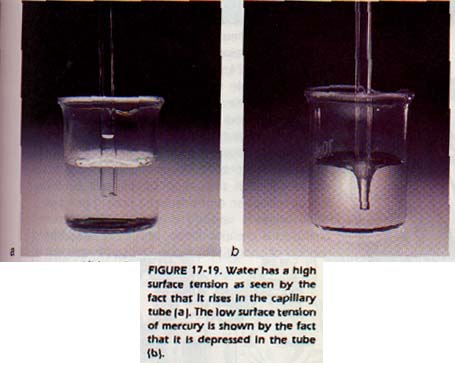
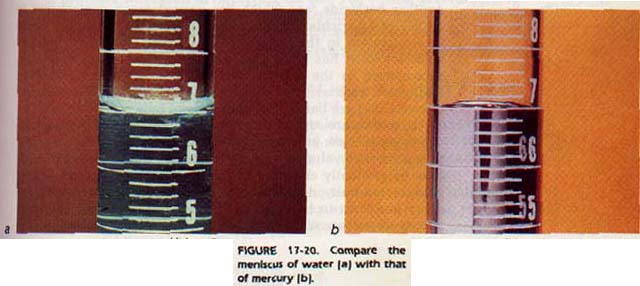
Capillarity is one method used for measuring surface tension. For example, water has a high surface tension at room temperature. It will rise quite readily in a capillary tube. Mercury, on the other hand, is depressed in a capillary tube as shown in Figure 17-19. It does not "wet" the glass of the tube. That is, there is not enough attractive force between the mercury and the glass to overcome the surface tension of mercury. Compare the meniscus of water and the meniscus of mercury in Figure 17-20. Can you think of an explanation for the difference in behavior?
SUMMARY
1. The vapor pressure of a substance is the pressure exerted by the gaseous phase in equilibrium with the liquid or solid phase.
2. At the melting or freezing point of any substance, the vapor pressure of the liquid and the vapor pressure of the solid are equal.
3. In a gas-liquid dynamic equilibrium, the number of molecules in both the liquid and gaseous phases remains constant.
4. If stress is applied to a system at equilibrium, the system tends to read just so that the stress is reduced (Le Chatelier's principle).
5. Sublimation is the change of state in which a substance passes directly from the solid to the gaseous state.
6. The boiling point of a liquid is the temperature at which the vapor pressure of the liquid is equal to the atmospheric pressure.
7. Evaporation is the process whereby molecules escape from the surface of a liquid or solid.
8. For every gas there is a critical temperature (Tc above which no amount of pressure will result in liquefying the gas. The critical pressure (Pc) is the pressure which will produce liquefaction at Tc . A low critical temperature indicates weak van der Waals forces between molecules.
9. A phase diagram is a graph showing the relationship between temperature, pressure, and physical state.
10. The triple point is that temperature and pressure at which all three states of a substance are in equilibrium.
11. Adding heat to a substance may change its kinetic energy or its potential energy. If the kinetic energy is increased, the temperature of the substance will rise. If the potential energy is raised, the substance will change state (melt or boil).
12. The heat of fusion is the heat required to melt 1 gram of a solid substance at its melting point. The heat of vaporization is the heat required to vaporize 1 gram of a liquid at its boiling point.
13. Compounds containing hydrogen bonded to fluorine, oxygen, or nitrogen have unusual properties. These properties occur because of the formation of hydrogen bond.
14. Ice is less dense than water, which is most dense at 4oC. The expansion of ice upon freezing occurs because of hydrogen bonding between the highly polar water molecules
15. Unbalanced forces account for the surface tension of liquids. Capillary rise of liquids in small tubes is due to surface tension.
More on Liquids:
For a PowerPoint presentation Click Here.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
![]() ...................................... Chem Tutor ....................................
...................................... Chem Tutor .................................... 
 Return to the Big Chem Page
Return to the Big Chem Page