Nature, Chemistry, and You
![]()
Nature, Chemistry, and You
![]()
The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
NATURE, CHEMISTRY, AND YOU
Throughout recorded history (and even before), people have tried to alter their environment to improve their way of life. Such "tinkering" has often had unexpected results.
Some of the earliest examples we have of people altering their environment involve farming. More than 4000 years ago, the Sumerians of the lower Tigris-Euphrates valley built a system of canals and dikes. This system was used to control yearly floodwaters and to carry water to crops in dry areas.
However, as the water flowed down from the mountains, it picked up salts in the hills. When it reached the fields, some of the water was used by the plants. The rest evaporated and left salts in the soil where they collected over the centuries.
The Babylonians, who next occupied the land, paid the price for such "tinkering."
When the early Egyptian pharaohs built the pyramids about 4500 years ago, they had to quarry huge amounts of stone. The Great Pyramid alone needed over five million metric tons of stone! In the process of obtaining this stone, the landscape was badly scarred.
In India, about 400 A.D., a method for making rust-resistant iron was discovered. This method was later used by the Persians and then by the Arabs. The fine "Damascus" steel in swords which were used in many "holy wars" was an unexpected later application of this Indian discovery.

About 600 A.D., the Chinese discovered an explosive mixture containing potassium nitrate. They used it to make fireworks for amusement. Five centuries later, this same mixture was being used as a gunpowder.
There is nothing good or bad about water, salt, stone, steel, or potassium nitrate. People determine whether these things are helpful or harmful. The people of Sumeria, India, and China could not have foreseen the damage their discoveries would bring. They did not intend for their work to lead to undesirable results. Their aim was simply to improve the quality of their lives.
1:1 LlMlTATlONS AND OPPORTUNlTlES
Today we face many problems resulting from past attempts to ''tinker" with nature. We have learned that we must plan for the future with care. However, planning for the future requires making choices.
Livable space on our planet is limited. Space travel is enormously expensive. It seems unlikely that people will colonize space stations in the near future. Therefore, we must use our existing resources wisely. An increasing world population leads to greater needs for housing, food, and water. Housing and farming both require suitable space.
How is the available space to be divided? How is the available water supply to be divided? Machines make our lives easier and more fun. However, machines need energy to run. The demand for energy is growing rapidly, yet our energy resources are limited. How should known energy resources be used? There is no single "best" answer to this question.
1:2 FlNDlNG OUT AND MAKING CHOlCES
People and their leaders make daily decisions which affect the use of the environment and natural resources. To make an intelligent choice, one must know the facts on each side of a question. The decision a person makes involves a value judgment. That is, the person must apply her own values and beliefs to the facts at hand.
Values vary from country to country. Moral and ethical standards differ from person to person. You may judge a person as "good" or "bad" on the basis of how that person behaves.
You are really comparing their behavior to your own set of standards. This kind of judgment is called a value judgment. The facts of nature, however, are neither good nor bad.
Established facts are the same for everyone. For example, table salt dissolves in water. A diamond is hard. Neither of these statements can be labeled as good or bad. Neither is the process of determining these facts good or bad.
To make an intelligent choice, one must be aware of the available facts. How we use facts involves value judgments. A collection of facts is neither good nor bad.
It is the way facts are used which may be good or bad. For example, scientists have learned the fact that sudden movements of the earth's crust can cause earthquakes. Can we hold scientists responsible for the destruction of life and property by an earthquake?
Scientists have also learned the fact that huge amounts of energy are released by changes in certain atomic nuclei. It is the use of this fact to make a nuclear bomb, not the fact itself, which involves a value judgment.
Even when the facts are known, making choices can be difficult. In making one choice, we decide against others. Usually, an advantage is traded for a disadvantage. For example, we may choose to develop more efficient car engines to save energy. However, this development will also mean more expensive cars.
We choose to ship oil in huge tankers in order to lower shipping costs. However, if one of these tankers is involved in an oil spill, the resulting damage to the environment is beyond measurement in dollars. Science cannot provide the values a person uses to make such choices.

Science deals with learning facts about the universe. The scientist uses many methods to try to obtain facts free from human bias. However, methods of learning facts and applying them cannot be freed from human values.
Science is always changing. Science is not a set of procedures or a certain group of people. It is not a collection of facts which never changes and should not be viewed as a subject forever a mystery to you.
Someday you may decide to pursue a career as a scientist and seek facts about our world. As a scientist, you will make observations. You will also hypothesize (make predictions based on your observations) and then experiment to test your hypotheses. In this way, you will add to the collection of facts that scientists have already recorded. This collection of facts is a product of science. The information it provides may help us make wiser choices in planning for our future.
1.3 CHEMISTRY
Chemistry is the study and investigation of the structure and properties of matter. Thousands of such studies have been made. As a result, certain properties are found to be related to the internal structure of matter. The first chemists were the Alchemists.

Knowledge of the relationship between structure and properties can be useful. For example, an engineer may tell a chemist that a new material with certain properties is needed for a job.
With this information, the chemist can predict what structure that material should have. For example, many new materials had to be developed for space exploration. These materials had to fit exacting specifications. An attempt was then made to produce the needed material.
A chemist may study many things. Such studies could be as different as the structure of the human brain or the bonding of rubber in a car tire. To make these studies, a chemist must be familiar with all of the sciences, including physics and mathematics. A chemist expresses the results of these studies as characteristics of the materials being examined.
Certain basic principles and facts relate the properties of materials to their structure. These basic facts and principles are the foundations of chemistry. Therefore, it is not necessary for a person to study the properties of all known materials in order to gain a knowledge of chemistry.

1:4 MATTER
All material is called matter by scientists. Matter may be as difficult to observe as the particles which produce the odor of perfume. It may be as easy to observe as a block of lead.
Matter is defined by scientists as anything which has the property of inertia. What is inertia? Inertia (in UHR shuh) is the resistance of matter to any change in motion. This change can be in either the direction or the rate of motion. or in both.
For example, suppose you are riding in a moving car. When the car is stopped suddenly, your body tends to continue to move forward. If the car makes a sharp turn, your body tends to continue to move in its original direction. Thus, you are thrown against the side of the car opposite from the direction of the turn. In both cases, your body is showing the property of inertia. All matter has the property of inertia.
1:5 ENERGY
In the study of science, an important concept to understand is energy. For many years, energy has been defined as work or as the capacity to do work. This definition is not always useful since energy takes so many forms. For instance, think about the battery-alternator system of a car.
As the starter switch is turned on, the chemical energy in the battery is converted to electrical energy. The car starts and chemical energy in the gasoline is converted into the energy of the moving car. As the crankshaft gains speed, its mechanical energy is transferred by belt and pulley to the alternator. In the alternator, the mechanical energy is converted into electric energy. This electric energy is transferred to the battery where it is converted to chemical energy.
The battery is thus "recharged." During this time, other kinds of energy such as heat and sound are also produced as by-products. At first, we shall define energy as a property of all matter. Under certain conditions, energy can be changed into work. We also know that energy can be converted from one kind to another. Energy can also be transferred directly from one particle of matter to another.
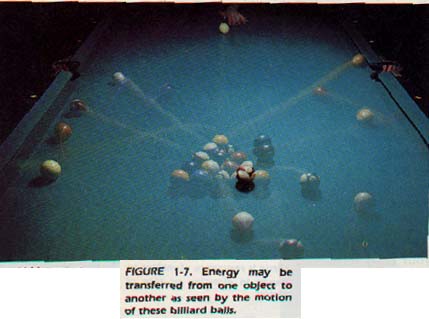
For example, when two billiard balls collide, energy is transferred from one ball to the other. With the exception of nuclear change, all such transfers of energy occur without an observable loss or gain in the total amount of energy.
An object or sample of matter, will have two general forms of energy: potential energy and kinetic energy.
Potential energy depends upon the position of the object with respect to another object.
Kinetic energy refers to the motion of one object with respect to another object. A book on a table has a greater potential energy relative to the earth than a book on the seat of a chair. The book on the table is further from the earth than the book on the chair. Therefore, it can do more work in falling to the floor than the book on the chair. An airplane traveling 700 kilometers per hour has a greater kinetic energy with reference to the earth than a bus traveling 88 kilometers per hour.
When two billiard balls collide, kinetic energy is transferred directly from one ball to the other. Often, energy is transferred between objects not in contact. An example is the transfer of energy from the sun to Earth. Energy being transferred through empty space is called radiant energy. Unlike potential energy and kinetic energy, radiant energy is not the property of an object. Light is a kind of radiant energy. All other types of energy (chemical, mechanical, electrical, etc.) are special cases or combinations of kinetic, potential, and radiant.
1:6 MATTER AND ENERGY
For years, scientists thought that the total amount of matter and the total amount of energy in the universe were each constant. They stated this belief in the form of two laws. These laws are the law of conservation of matter and the law of conservation of energy.
The law of conservation of matter states that natter is always conserved. This statement means that the total amount of matter in the universe remains constant. Matter is neither created nor destroyed. It is only changed in form.
The law of conservation of energy states that energy is always conserved. This statement means that the total amount of energy in the universe remains the same. Energy is neither created nor destroyed. It too, is only changed in form. Albert Einstein, in the early 1900's, showed that, in fact, matter can be changed to energy. He also showed that energy can be changed to matter. Einstein expressed this relationship in his famous equation:
E = mc2
In this equation, E is energy, m is mass, and c is the speed of light (300,000 km/sec).
According to Einstein's equation, mass and energy are equivalent. Thus we see that the two conservation laws are really just one law. This law is known as the law of conservation of matter-energy.
Because mass is a measure of the amount of matter, this law is usually called the law of conservation of mass-energy. The law of conservation of mass-energy states that mass and energy are always conserved and that their sum cannot be increased or decreased.
Mass and energy can, however, be changed from one to the other. Changes of energy to mass and mass to energy are observable only in nuclear reactions. In our laboratory work and in our discussions, we will always assume the original laws of conservation of matter and energy to be correct.

SUMMARY
1. For thousands of years, people have altered their physical environment. Their work has led to both helpful and harmful results.
2. Today, people face many decisions which will affect their environment and the use of natural resources. In order to make intelligent decisions, people must obtain the facts related to the decision to be made.
3. The function of science is to provide the facts needed to make informed, intelligent decisions.
4. Chemistry is the science of materials. A chemist studies the properties and structures of materials.
5. Matter is anything with the property of inertia. Inertia is the resistance of an object or particle to a change in either its direction of rate of motion, or in both.
6. The energy of an object consists of potential energy and kinetic energy.
7. Energy being transferred through empty space between objects is in the form of radiant energy.
8. Under certain conditions, any form of energy can be transferred from one object to another or made to do work on an object.
9. The law of conservation of mass-energy states that mass and energy are always conserved and that the sum of mass and energy is always the same.
Ah Yaz Indeed!
............... First Semester Chapters 1-18
............... Second Semester Chapters 19-30
 Chemistry *** Class Notes & Overheads ***
Chemistry *** Class Notes & Overheads ***
 Return to the Big Chem Page
Return to the Big Chem Page