Phases of Matter

Phases of Matter

THE STRUCTURE OF MATTER
7.1 Early Theories When a lump of sugar is crushed into smaller pieces, each of these pieces is still a particle of sugar. Only the size of the piece of sugar is changed. If the subdividing process is continued by grinding the material into a fine powder, the results are the same.
Even when the sugar is dissolved in water and the pieces are too small to be seen with a microscope, the taste of the sugar is retained. Evaporation of the water returns the solid sugar's other identifying properties, such as color, crystalline shape, and density.
Observations such as these, together with the quantitative evidence supporting the laws of definite and multiple proportions, which you may have already studied in chemistry, lead to these conclusions about the nature of matter:
1. Matter is composed of particles.
2. The ultimate particles of matter are extremely small.
As early as 400 B.C., Greek philosophers formulated ideas about the ultimate composition of matter. Democritus (460-370 B.C.) believed that matter is indestructible and that the subdividing process mentioned above would reach a limit beyond which no further separation is possible. He called these ultimate particles atoms, after the Greek word atomos meaning "indivisible." This idea was largely the result of rational thinking. Democritus had no direct evidence to back up his concept of atoms.
The Greek philosopher Aristotle (384-322 B.C.) suggested that all matter can be reduced to four basic elements: air, earth, fire, and water. The reasoning that was used to support this idea was often quite complex, but it was a beginning in the attempt to list the ultimate particles of universe.
It was a long time before the idea of atoms and particles was actively studied again. Near the beginning the 19th century, the English scientist John Dalton (1766-1844) conducted a series of experiments with gases added greatly to our knowledge of the nature of atoms. Dalton's atomic theory explained the mechanisms of know reactions between substances and made it possible to extend the list of known elements.
The atomic theory has been modified and many times since Dalton's day. Almost two hundred years of investigation have added much information to the knowledge of the makeup and properties of atoms, the way in which they interact, and the nature of the compounds they form.
Modern theory includes data concerning the mass and size of atoms as well as the relationships among atoms. Experiments have also revealed the fact that atoms are not ultimate particles after all. A whole array of subatomic particles presently being studied by scientists. (See Chapters 23-25.
7.2 Molecules A molecule is the smallest chemical unit of substance that is capable of stable, independent existence. In the example in the previous section, the smallest particle sugar that retains any identifying properties of the substance is a molecule of sugar.
Not all substances, however, are composed of molecules. Some substances are composed of electrically charged particles known as ions.
To get an idea of the extremely small size of molecules imagine a drop of water magnified until it is as large as earth. With this tremendous increase in size, a single molecule of water would be about one meter in diameter. Simple molecules, like that of water, is about 3 x 10-10 or 3 angstroms (A) in diameter.
Molecules of more complex substances may have of more than 200 A. The electron microscope, which is capable of magnifications of several million times, can used to photograph some of these "giant" molecules.
7.3 Atoms If a molecule of sugar is analyzed, it is found to consist of particles of three simpler kinds of matter: carbon, hydrogen, and oxygen. These simpler forms of matter are called elements.
An atom is the smallest unit of an element that can exist either alone or in combination with other atoms of the same or different elements. A molecule of sugar is made up of atoms of carbon, hydrogen, and oxygen.
Since atoms make up molecules, atoms are usually smaller than molecules. The smallest atom, an atom of hydrogen, has a diameter of about 0.6 A. The largest atoms are a little more than 5 A in size. The hydrogen atom is also the lightest atom. It has a mass of 1.673559 x 10-27 kg. The most common uranium atom, which is one of the heaviest atoms, has a mass of 3.952989 x 10-25 kg. There is about a tenfold range in the sizes of atoms and about a 250-fold range in their masses.
In 1970 the American scientist Albert Crewe (b. 1927) took the first photos of atoms. In 1976 Dr. Crewe obtained black and white movies of atoms. Two years later, Crewe used an electron microscope to take color movies of atoms.
Even with the use of scientific notation, it is difficult to express conveniently the masses of individual atoms. Consequently, scientists express the atomic mass of an atom in atomic mass units (u). One u is equal to 1.6605655 x 10-27 kg. This is the mass of a carbon-12 atom. Carbon-12 is the most abundant form of carbon atoms. In other words, scientists use carbon-l2 as the standard of mass for atoms.
In atomic mass units, the atomic mass of the most abundant form of hydrogen is 1.007825 u, while that of the most abundant form of uranium is 238.0508 u. You will notice that atomic masses are known very accurately. The integer nearest to the atomic mass is called the mass number of an atom. The mass number is represented by the symbol A. Thus for the common hydrogen atom, A = 1; for the common uranium atom, A = 238.
The concept of atoms is very useful in the study of chemical reactions. When substances interact chemically, atoms are regrouped, but the number of the various kinds of atoms does not change during the reaction.
7.4 Kinetic Theory of Matter So far in our study of physics, we have been concerned almost entirely with the behavior of solid particles of matter. A solid is one of the three phases (or states) of matter-- the solid phase, the liquid phase, and the gaseous phase.
In the description of matter phase indicates the way in which particles group together to form a substance. The structure of a substance can vary from compactly arranged particles to highly dispersed ones.
In a solid, the particles are close together in a fixed pattern. In a liquid, the particles are not usually as close together as in a solid and are not held in any fixed pattern. In a gas, the average separation of the particles is relatively large, and as in a liquid, the particles are not held in any fixed pattern. (Frequently the term vapor is used to describe a gas that under ordinary conditions of temperature and pressure is in the liquid phase.)
Both liquids and gases are also called fluids (from Latin meaning "to flow"). To explain the motions of molecules and the energy molecules possess, particularly in the gaseous phase, scientists have developed the kinetic theory of matter. Two basic concepts of this theory are:
1. The molecules of a substance are in constant motion. The amount of motion depends upon the average kinetic energy of the molecules; this energy depends upon the temperature.
2. Collisions between molecules are perfectly elastic (except when chemical changes or molecular excitations occur).
7.5 Forces Between Molecules The forces required to pull a solid apart are generally much greater than the forces required to separate a similar amount of liquid. Liquids separate into drops. The forces of attraction between the molecules of liquids are not as great as the attractive forces of solids.
Most liquids occupy a larger volume than the same mass of solid. Since the size of a molecule of a substance in one phase does not differ appreciably from its size in another phase, molecules of liquids must be farther apart than molecules of solids.
In a gas, molecules separate from each other spontaneously; this accounts for the fact that a gas occupies a volume about 1000 times that of an equal mass of liquid. This spontaneous separation of gas molecules indicates that the kinetic energy of the molecules is great enough to keep them separated. We may conclude that forces between molecules decrease as the distance between them increases.
Solids and liquids are not easily compressed. Apparently, when molecules in solids and liquids are pushed closer together than their normal spacing, they repel each other. As the molecules are pushed still closer together, the repulsive forces become greater.
Intermolecular forces (van der waals) are mainly electric. They are about 1029 times as strong as the gravitational forces between molecules at the typical distances found in solids and liquids. Thus gravitational forces between molecules are negligible in comparison with intermolecular forces. Intermolecular forces are small compared to the weight of objects we can see and handle; however, the masses these intermolecular forces act on the masses of molecules are small, too.
These forces can impart instantaneous accelerations 1014 times the acceleration of gravity. Such accelerations last only a very short time since one molecule, so accelerated, moves quickly out of the range of another.
Figure 7-3 shows how the force of interaction between molecules varies with the distance between their centers. If we imagine one molecule to be fixed at the intersection of the axes, the other molecule will be repelled until the distance of separation is such that their outer charges do not overlap.
This condition occurs where no net force acts between the molecules. The distance is often called the equilibrium distance. This distance (about 2.5 x 10-10 meter) is, therefore, the distance between the centers of two "touching" molecules and is the diameter of a single molecule.
As the separation between the molecules increases, the force of attraction between opposite charges first increases and then approaches zero. Different molecules have different sizes and charge configurations, but they always show the qualitative behavior indicated by Figure 7-3.
In the solid phase, molecules vibrate about the equilibrium position. They do not have enough energy to overcome the attractive force. The equilibrium positions are fixed. In a liquid the molecules have greater vibrational energy about centers that are free to move, although the average distance between the centers of the molecules remains nearly the same.
The average distance of separation between gas molecules is considerably greater than the range of intermolecular forces, and the molecules move in straight lines between collisions.
THE SOLID PHASE
7.5 The Nature of Solids Solids have definite shapes and definite volumes. Scientists usually describe solids as either crystalline or amorphous. Crystalline solids have a regular arrangement of particles; amorphous solids have a random particle arrangement.
In addition to the forces that bind particles of a solid together, the motion of particles of a solid is an important consideration. Particles of a solid are held in relatively fixed positions by the binding forces. However, they do have a vibratory motion about their fixed positions. The amplitude of their vibration and their resulting vibratory energy are related to the temperature of the solid. At low temperatures the kinetic energy is small, at higher temperatures it is larger.
Diffusion is the penetration of one type of particle info a mass consisting of a second type of particle. If a lead plate and a gold plate are in close contact for several months, particles of gold may be detected in the lead, and vice versa. This demonstrates that even solids may diffuse. Diffusion is slow in solids because of the limited motion of the particles and their close-packed, orderly arrangement.
7.7 Cohesion and Adhesion The general term for the force of attraction between molecules of the same kind is cohesion. Cohesion, the force that holds the close-packed
molecules of a solid together, is a short-range force. If a solid is broken, layers of gas molecules from the air cling to the broken surfaces. These gas molecules prevent the
rejoining of the solid surfaces. The molecules of the broken surfaces are not close enough to have sufficient attraction to hold. However, if the surfaces of two like solids are polished and then slid together, cohesion causes the solids to stick together.
Molecules of different kinds sometimes attract each other strongly. Water wets clean glass and other materials. Glue sticks to wood. The force of attraction between molecules of different kinds is called adhesion. The forces of cohesion and adhesion have definite values for specific molecules.
7.8 Tensile Strength Several properties of solids depend on cohesion; one of these is tensile strength. Suppose two wires of the same diameter, one copper and one steel, are put in a machine that pulls the wires until they break. When tested in this manner, steel wire proves stronger than copper wire of the same diameter. Therefore we say that steel has a higher tensile strength than copper. The tensile strength of a material is the force per unit cross-sectional area applied perpendicularly to the cross section that is required to break a rod or wire of that material. See Figure 7-5 and Appendix B, Table 7. Tensile strength is a measure of cohesion between adjacent molecules over the entire cross-sectional area.
7.9 Ductility and Malleability If a metal rod can be drawn through a small opening, or die, to produce a wire, the metal is said to be ductile (duk-til) or to possess ductility. As the metal is pulled through the die, pressure decreases its diameter and increases its length and the rod becomes a wire. See Figure 7-6(A). In one industrial application of ductility, more than 1OOO meters of wire are formed per minute in a device in which the pressure is exerted by a fluid, and the wire never touches the solid die. Platinum is so ductile that a single gram of the metal can be drawn into a wire almost 600 kilometers long. WOW!
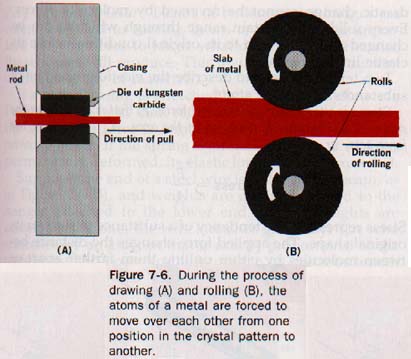
Metals that can be hammered or rolled into sheets are said to be malleable (mal-ee-uh-bul) or to have malleability. During the hammering or rolling, the shape of the metal is greatly changed. See Figure 7-6(B).
In the process of hammering, rolling, or drawing, layers of atoms of a metal are forced to slide over one another, thus changing their positions in the crystal pattern. Since the cohesive forces are strong and the atoms do not become widely separated from each other during their rearrangement, the metal holds together while its shape is being changed. Silver, gold, platinum, copper, aluminum, and iron are all highly malleable and ductile.
7.10 Elasticity When opposing forces are applied to an object, the size and shape of the object are changed. The ability of an object to return to its original size or shape when external forces are removed is described as its elasticity. However, there is a limit beyond which the change produced by the applied forces does not disappear when the forces are removed. Ductility and malleability are properties of substances that can undergo such permanent changes without fracturing. When a substance is on the verge of becoming permanently changed, we say it has reached its elastic limit.
At the elastic limit, molecular forces are overcome to such an extent that particles slide past each other, and the shape of the material is permanently changed. Such a drastic change it cannot be reversed by molecular forces. Every solid has a certain range through which it can be changed and yet return to its original condition before its elastic limit is reached.
Two terms are used to describe the elastic properties af substances: stress and strain. Stress is the ratio of the internal force, F, that occurs when the shape of a substance is changed to the area, A, over which the force acts, or
stress = F/A
Stress represents the tendency of a substance to recover its original shape. The applied force changes the distance between molecules by either pulling them farther apart or pushing them closer together. When the force is removed, molecular forces restore the molecules to their normal spacing.
Strain is the relative amount of deformation produced in a body under stress. There are various kinds of strain, but its measure is always an absolute ratio-- a number without units. When a rod is placed under tension, it stretches. The ratio of the increase in length to the original length is called the elongation strain and is expressed as ΔL / L.
Linear compression strain is the reverse of elongation, as shown in Figure 7-7. Elongation and compression are accompanied by small changes in the cross-sectional area, but their effect is small in situations where elastic limits are not exceeded. In an elongation strain, the particles have been moved in a direction perpendicular to the area over which the forces act.
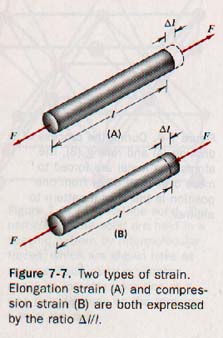
In a shear strain, the particles move in a direction parallel to the area over which the forces act.
A cube will take the shape of a rhombic prism when shear forces are applied to it. The measure of shear is the ratio of the amount the top of the cube is moved to the side to the length of one side of the cube. This ratio, shear, is expressed as the tangent the angle through which the oblique edges of the imaginary cube have been rotated from their original direction, as shown in Figure 7-8.
Volume strain is the ratio of the decrease in volume to the volume before the stress was applied.
Flexure (bending) and torsion (twisting) are conditions of elongation and compression strains. A beam bent into a plane curve undergoes compression one side and elongation on the other side. The layer of material down the center of the beam undergoes neither compression nor elongation.
7.11 Hooke's Law Beams of buildings and bridges are often subjected to varying forces or stresses. It is important for engineers to know what deformation, or strain, these forces will produce. This involves the measurement of the elasticity of materials.
If a coiled spring is stretched by a weight, as shown in Figure 7-9(A), and then returns to its original form after the stretching force is removed, the spring is said to be perfectly elastic. If the spring is stretched too far, it remains permanently deformed; its elastic limit has been exceeded.
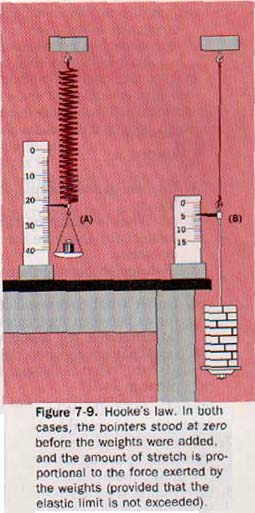
Suppose one end of a steel wire is fastened to a beam, as in Figure 7-9(B), and weights are gradually added to the hanger attached to the lower end. As the weights are added one by one, the wire stretches gradually. The wire stretches by an amount that is exactly proportional to the weight pulling on it, and it returns to its previous length when the weight is removed. If more weights are added, the elastic limit is eventually reached. Then when the weights are removed, the wire remains deformed; it does not return to its original length.
By making such measurements, the English philosopher and scientist Robert Hooke (1635-1703) found that the amount of elongation in elastic solids is directly proportional to the deforming force provided the elastic limit is not exceeded. The elongation also depends on the length and cross-sectional area of the wire or rod. All these facts were combined by Hooke into one simple law. Hooke's law states that within certain limits strain is directly proportional to stress.
The value of the ratio stress/strain is different for different solids. However, the ratio is constant for a given substance, even when the substance has different shapes. This ratio gives us a means of comparing the elasticity of various solids. The numerical value of Hooke's law is called Young's modulus, Y, and is defined by the equation
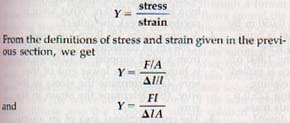
THE LIQUID PHASE
7.12 The Nature of Liquids In 1827 the English botanist Robert Brown (1775-1858) put some pollen grains in water and placed a bit of this suspension on a small slide. When he examined the suspension through a he found that the pollen grains moved in a random way.
The path of a single particle resembled shown in Figure 7-10. This so-called Brownian movement caused by the continual bombardment of the sub-particles by molecules of the surrounding liquid. Brownian movement shows that molecules of a liquid are constant, rapid, and random motion. This is in keeping the kinetic theory of matter described in Section 7.4.
Liquids diffuse. This can be shown by the experiment in 7-11. Enough concentrated copper(II) sulfate solution poured into a tall cylinder to form a layer several centimeters deep. A flat cork is floated on the surface of solution. Water is carefully poured through a funnel onto the top of the cork.
The water flows around the of the cork and spreads out over the surface of the sulfate solution, producing two distinct layers. water "floats" on the copper(II) sulfate solution because water has a lower density. After the cylinder sits for a few days, the interface between the layers is indistinct. Some of the copper(II) sulfate solution diffused into the water above, and some of the water molecules diffused into the copper(II) sulfate solution below. Though weeks may pass before the diffusion is complete we can see that diffusion does occur in liquids despite the force of gravity. Because of the slightly more open molecular arrangement and greater molecular mobility than that of solids.
7.l3 Cohesion and Adhesion If you push a spoon into a jar of honey and then pull it out, a certain amount of force is needed to pull apart the molecules of the honey. If you lick the honey from the spoon, you find that force is required to pull the molecules of the honey away from the molecules of the spoon.
These are examples of cohesion within a liquid and adhesion between a liquid and a Solid. If a clean glass rod is dipped into water and then removed, some of the water clings to the glass rod. We saw that the water wets the glass. The adhesion of water molecules to glass must therefore be greater than the cohesion between water molecules.
If the glass rod is dipped into mercury and then moved, the mercury does not cling to the glass the cohesion between mercury molecules is greater their adhesion to glass.
Cohesive forces vary in liquids. They are usually smaller in liquids than in solids.
If you examine the surface of water in a glass you will find that it is not completely level. It is slightly concave when viewed from above. The edge of surface where water comes in contact with the glass is lifted a little above the general level.
The crescent-shaped face of a liquid column is called the meniscus. The water rises at the edge because adhesion between water and glass is greater than cohesion between water molecules. If the container contains mercury instead of water, edges of the liquid are depressed and the surface is slightly convex. In this case, cohesion between mercury is greater than adhesion between mercury and glass. Cohesion is also responsible for a property of called viscosity. Viscosity is the ratio of shear stress to the of change of shear strain in a liquid or gas.
The viscosity of fluid determines the rate of flow of the fluid. Viscosity is dependent on temperature. As the temperature increases the viscosity of gases increases while the viscosity of liquids decreases. This explains the expression "as slow molasses in January."
Modern motor oils are specially formulated to minimize the effect of temperature on viscosity. Under normal conditions, gases are much less viscous than liquids.
7.14 Surface Tension Have you ever seen a sewing needle or a razor blade floating on the surface of water? Even though they are about seven times as dense as water, if they are placed carefully on the surface, they remain there. A close look at the water surface shows that the needle or razor blade is supported in a hollow in the water surface, as seen in Figure 7-12.
The water acts as though it has a thin, flexible surface film. The weight of the needle or razor blade is counterbalanced by the upward force that is exerted by the surface film. This property of liquids is due to surface tension.
All liquids show surface tension. Mercury has a very high surface tension. In many liquids the surface film is not as strong as that of water or mercury. Part of the cleaning action of detergents is due to their ability to lower surface tension.
This makes it possible for the water and detergent to penetrate more readily between the fibers of the articles being cleaned and the dirt particles. Since particles in a liquid attract similar liquid particles that are nearby, they move as close together as possible. Hence the surface will tend to have a minimum area.
The effect of this attraction is to make the liquid behave as if it were contained in a stretched elastic skin. The tension in this "skin" is the surface tension. When a force acts on a liquid surface film and distorts that film, the cohesion of the liquid molecules exerts an equal and opposite force that tends to restore the horizontal surface. Thus the weight of a supported needle produces a depression in the water surface film that increases the area of the film.
In order to restore the surface of the liquid to its original horizontal condition, the cohesion of the water molecules exerts a counterbalancing upward force on the needle. Surface tension produces contraction forces in liquid films. A liquid film has two free surfaces on which molecules are subject to an unbalanced force toward the inside of the film. Thus both free surfaces tend to assume a minimum area.
The contraction of the film can be demonstrated by the device shown in Figure 7-13. A wire ring containing a loop of thread is dipped into a soap solution causing a film to form across the ring. If the film inside the loop of thread is broken with a hot wire the unbroken film outside the loop contracts and pulls the thread equally on all sides to form a circle.
Surface tension causes a free liquid to assume a spherical shape. A free liquid is one that is not acted upon by any external force. This condition can be approximated by small drops of mercury on a table top, as shown in Figure 7-14. A sphere has the smallest surface area for a given volume. The unbalanced force acting on liquid surface molecules tends to pull them toward the center of the liquid, reducing the surface area and causing the liquid to assume a spherical shape.
Since the cohesive force between mercury molecules is great and mercury has a very high surface tension, small drops of mercury are almost spherical. Larger drops of mercury, on which the effect of the force of gravity is greater, are noticeably flattened.
7.5 Capillarity When the lower ends of several tubes are immersed in water, the water will rise to the same height in each tube only if the tubes have large enough diameters so that the centers of the menisci are relatively flat.
Water does not rise to the same height in tubes of small diameters. The height to which it rises increases as the diameter of the tube decreases. See Figure 7-15(A). When mercury is used, the depression of the surface is greater as the diameter of the tube is reduced. See Figure 7-15(B). This elevation or depression of liquids in small-diameter tubes is called capillarity.
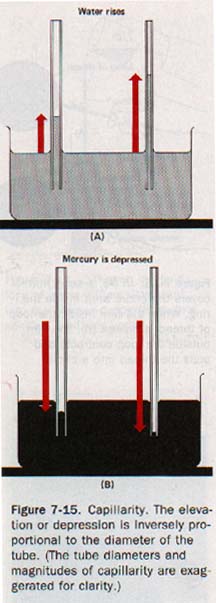
Capillarity depends on both adhesion and surface tension. Adhesion between water and glass causes water to creep up the glass walls and produce a concave surface; surface tension tends to flatten this surface by contraction.
The combined action of these two forces raises the water above its surrounding level. The water level rises until the upward force is counterbalanced by the weight of the elevated liquid.
Experiments have verified the following:
(1) Liquids rise in capillary tubes they wet and are depressed in tubes they do not wet.
(2) Elevation or depression is inversely proportional to the diameter of the tube.
(3) The elevation or depression decreases as the temperature increases.
(4) The elevation or depression depends on the surface tension of the liquid.
7.16 Melting The change of phase from a solid to a liquid is called melting. Melting involves the breaking of bonds between the particles of a solid. The temperature at which this change occurs is called the melting point. Pure crystalline solids have definite melting points, and different solids have different melting points.
When a substance changes from a liquid to a solid, it is said to freeze. The temperature at which freezing occurs is known as the freezing point. For pure crystalline substances, the melting point and the freezing point are the same temperature at any given pressure.
Noncrystalline solids, like paraffin, have no definite melting point. When they are heated, they soften gradually. The temperature at which noncrystalline solids first and the temperature at which they flow freely are often greatly different.
In order for a solid to melt, energy must be supplied to it. This energy increases the energy of the particles of the solid and gives them the freedom of motion characteristic of the particles of a liquid.
As the temperature of a solid increases, the vibrations of its particles increase in amplitude; thus more and more potential energy is stored in the average stretching of the bonds between the particles. Finally a point is reached at which the bonds between the particles cannot absorb any more energy without breaking. Thus crystalline solids have a definite melting point.
When the liquid formed by melting a crystalline solid cools to a certain temperature, the energy of the liquid particles is reduced and the forces between the particles draw them into fixed positions in a crystal. Thus a liquid that forms a crystalline solid freezes at a definite temperature.
All the energy supplied to a substance during melting is used to increase the potential energy of the particles in changing from a crystal structure to a liquid. The kinetic energy of the particles does not change. Since average kinetic energy depends on temperature, the temperature is unchanged during the melting process.
Usually, the particles of noncrystalline solids are held together by forces of attraction and by the physical entanglement of long-chain molecules. The bonding combination is not of such definite strength that the bonds are broken when the particles acquire a fixed amount of energy. The energy required to overcome these bonds varies with the extent of the bonding and entanglement of each molecule.
As they are heated, noncrystalline substances soften at a lower temperature first. At some higher temperature, they flow freely. Similarly, as they are cooled, the molecules become bonded at various kinetic energies, and the liquid does not solidify at a definite temperature.
The degree of separation of particles of a substance is different in the solid and liquid phases because of the difference in the potential energy of the particles. If melted paraffin is poured into a vessel and allowed to harden, the center becomes indented, or depressed, as shown in Figure 7-17(A). The paraffin cools and contracts as it solidifies. Both kinetic energy and potential energy are lost in the process.
The loss of kinetic energy is indicated by the decrease in temperature. Loss of potential energy permits the particles to move closer together and take up less space. Almost all substances behave in this manner; the particles of most substances are closer in the solid phase than in the liquid phase.
Water is the most important exception to the rule that a substance contracts when it changes from a liquid to a solid. See Figure 7-17(B). When an ice cube tray is placed in the freezing compartment of a refrigerator, the level of the water in the sections of the tray is uniform. When the ice cubes are formed, however; each one has a slightly raised spot in the center.
The volume occupied by ice is about 1.1 times that occupied by the water from which it was formed. The force of expansion when water freezes is enormous. Some calculations have placed the pressure as high as 83,000 newtons per square centimeter! WOW!
Antifreeze is used in water-cooled cars that are driven in areas where the atmospheric temperature drops below the freezing point of water. As its name suggests, a mixture of antifreeze and water freezes at a lower temperature and does not expand significantly in freezing if the mixture is sufficiently concentrated.
Bismuth and antimony are two metals that expand rather than contract when they solidify. Substances such as ice, bismuth, and antimony have open crystal structures in which the particles are more widely separated than they are in the liquid phase. That is why the solid occupies a larger volume than the liquid.
7.17 Effect of Pressure on the Freezing Point In substances that contract when they solidify, like the paraffin in Figure 7-17(A), the molecules of the solid are closer together than the molecules of the liquid. If additional pressure is exerted, such a liquid can be made to solidify at a higher temperature than its normal freezing point under atmospheric pressure.
An increase in pressure raises the freezing (melting) point of most substances. Some of the rock in the interior of the earth is hot enough to melt at normal pressure, but most of it remains solid because of the tremendous pressure. If the pressure is released, as when a volcano erupts, more of the rock melts and forms lava
An increase in pressure has the opposite effect on the freezing point of a substance like water that expands as it freezes. In such a substance the molecules are farther apart in the solid than they are in the liquid. Since an increase in pressure makes formation of the solid more difficult, the freezing point is lowered if the pressure is raised.
We can illustrate this effect by suspending two weights over the surface of a block of ice by means of a strong wire. See Figure 7-18. The pressure of the wire on the ice lowers the melting point of the ice immediately below the wire. If the surrounding temperature is above the new melting point, this part of the ice melts, and the molecules of water are forced upward around the wire. When they reach a spot above the wire, the pressure returns to normal, the melting point rises, and the water freezes again:
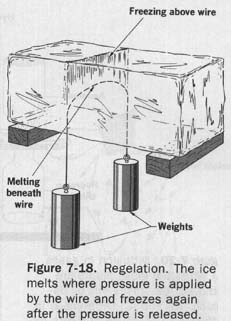
When the wire is embedded in the ice, the extra heat needed to melt the ice below the wire is supplied by the freezing of the water above the wire. In this way, the wire may cut its way through the block of ice, and yet leave the ice in one piece. This process of melting under pressure and freezing again after the pressure is released is called regelation (reejeh-lay-shun).
When the pressure of ice-skate blades on ice is sufficient to melt the ice at its existing temperature, the blades slide along with very little friction on a thin layer of water. However, if the ice is so cold that the pressure of the skate blades cannot melt the ice, the blades are retarded by the higher friction of steel on ice, and skating is difficult.
7.18 Effect of Solutes on the Freezing Point The freezing point of a liquid is lowered whenever another substance is dissolved in it. The extent of the lowering depends on the nature of the liquid, the nature of the dissolved substance, and the relative amounts of each.
The greater the amount of the solute in a fixed amount of liquid, the lower the freezing point of the liquid. We apply this principle when we use rock salt to prevent the formation of ice on roads and sidewalks, and to melt the ice that has formed. The dissolved substance interferes with crystal formation as the liquid cools.
Before crystals form, the kinetic energy of the liquid particles must be reduced to a level below that at which they normally crystallize.
THE GASEOUS PHASE
7.19 The Nature of Gases The following properties of gases show that gas molecules move independently of each other at high speed:
1. Expansion. A gas has neither a definite shape nor a definite volume; it expands and completely fills any container. This shows that gas molecules are independent particles.
2. Pressure. An inflated balloon may burst from the force that the air inside exerts on the balloon's inner surface. This force is a result of the continual bombardment of the inside surface by billions of moving molecules. If we increase the number of molecules within the balloon by blowing more air into it, the number of collisions against the inside surface increases, the pressure on the inside surface increases, and the balloon expands
.3. Diffusion. Hydrochloric acid is a water solution of the high-density gas hydrogen chloride; ammonia water is a water solution of the low-density gas ammonia. When these gases react chemically, they form a cloud of fine, white particles of solid ammonium chloride.
Suppose we put a few drops of hydrochloric acid in a warm bottle and an equal amount of ammonia water in a second warm bottle. We then cover the mouth of each bottle with a glass plate and invert the bottle containing ammonia over the one containing hydrogen chloride, as shown in Figure 7-20(A). After the bottles have stood this way for a minute or two, we remove the glass plates so that the bottles are now mouth-to-mouth. The formation of white smoke indicates that the less dense ammonia descends and mixes with the more dense hydrogen chloride.
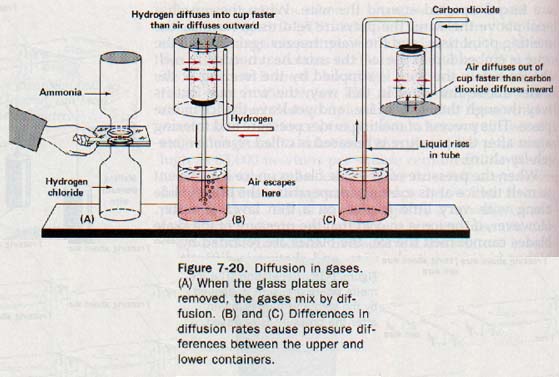
The hydrogen chloride also rises and mixes with the ammonia in the upper bottle. The movement of each of these gases is opposite to that which would be caused by the density of the gases The movement must be due to molecular motion. Thus diffusion of gases is evidence of the rapid movement of gas molecules.
Gases also diffuse through porous solids. In Figure 7-20(B), an inverted, unglazed earthenware cup is closed with a rubber stopper through which passes a glass tube that dips into a red liquid. When a low-density gas, such as hydrogen, is led into an inverted beaker placed over the cup, air immediately begins to bubble through the red liquid. Evidently the lighter molecules of hydrogen move in through the porous walls of the cup faster than the heavier molecules in the air move out. Thus there is an accumulation of hydrogen molecules inside the cup.
This accumulation increases the pressure inside the cup, thus pushing the liquid down the tube and forcing some of the air and hydrogen mixture in the tube to escape.
In Figure 7-20(C), a porous cup is surrounded with a dense gas, such as carbon dioxide. Since carbon dioxide is denser than air, the apparatus must be modified; the beaker surrounding the porous cup must be positioned with the open side up. When the beaker is filled with carbon dioxide, molecules from the air flow out through the porous cup faster than carbon dioxide molecules enter.
This difference reduces the pressure inside the cup, and the red liquid rises in the tube. Thus we see that there is an inverse relation between the rate of diffusion of a gas and its density.
Diffusion of gases through porous solids is an important process. Such diffusion occurs through the membranes of plants, animals, and humans, allowing oxygen to reach living cells and carbon dioxide to escape.
7.20 Vaporization When ethoxyethane (ether) is placed in a shallow dish, within a short time the quantity of liquid decreases and the odor of ether becomes quite strong near the dish. Apparently molecules of ether liquid become molecules of ether vapor and mix with molecules of the gases in the surrounding air.
In a similar manner, but at a much slower rate, paradichlorobenzene (mothaballa) left out in the air become smaller and smaller and eventually disappear while their characteristic odor is noticed in the air nearby. In this case, molecules of the solid moth balls turn into vapor and diffuse into the surrounding air.
These are two examples of vaporization. See Figure 7-21.
As in the case of melting (Section 7.16), vaporization is a constant-temperature process. All the energy supplied during vaporization goes to increase the potential energy of the particles.
The average kinetic energy of the particles is unchanged. The particles of all liquids and solids have an average kinetic energy that depends on the temperature of the liquid or solid. However, because of random collisions and vibratory motion, some particles have energies higher than average and others have energies lower than average.
When the particle on the surface of a liquid or solid acquires enough energy to overcome the forces that hold it as part of the substance, the particle escapes and becomes a particle in the vapor phase. When vaporization occurs from liquids it is known as evaporation. When vaporization occurs from solids, it is known as sublimation. Sublimation is a direct change from solid to vapor without passing through the liquid phase.
The energy of the particles that evaporate from a liquid is obtained either from the surrounding atmosphere from the remaining liquid. In either case, evaporation has a cooling effect on the environment. When perspiration from the skin, the process cools the skin. When rubbing alcohol is applied to the skin, the cooling effect ls even greater because alcohol evaporates more rapidly than water.
Since the rate of evaporation or sublimation depends on the energy of the particles undergoing the change and in turn their energy depends upon their temperature, evaporation and sublimation occur more rapidly at higher temperatures and more slowly at lower temperatures.
7.21 Equilibrium Vapor Pressure A bell jar covering a container of water has as many molecules of the gases of the air within the bell jar as there are in an equal volume of air outside it. The pressure (force per unit area) exerted by the gas molecules on the inside walls of the bell jar is the same as the pressure that such molecules exert on the outside walls.
When a molecule at the surface of the water inside the bell jar acquires sufficient kinetic energy, it escapes and becomes a water vapor molecule. As the water evaporates, water vapor molecules mix with the gas molecules in the bell jar. These water vapor molecules collide with gas molecules, with the walls of the bell jar, with the outside surface of the container of water, and with the surface on which the bell jar rests.
They can also touch the water surface, be held by it, and become molecules of liquid again. The conversion of molecules of vapor to molecules of liquid is called condensation.
Eventually the rate of evaporation equals the rate of condensation, and a condition of equilibrium prevails. At equilibrium, evaporation and condensation do not cease; they occur at the same rate. The number of water vapor molecules in the air in the bell jar remains constant. At equilibrium, the space above the water in the vessel is said to be saturated with water vapor.
The collision of water vapor molecules against the walls of the bell jar increases the pressure on the bell jar so that it exceeds the pressure exerted by the gases of the air. Added pressure exerted by vapor molecules in equilibrium with liquid is called equilibrium vapor pressure.
Since the kinetic energy of the particles of the liquid depends on both the temperature and mass of the particles, the equilibrium vapor pressure of a liquid depends on the composition as well as the temperature of the liquid. As the kinetic energy of the particles increases, so does the vapor pressure.
The ratio of the water vapor pressure in the atmosphere to the equilibrium vapor pressure at that temperature is called the relative humidity. Relative humidity is usually expressed as a percentage. For example, suppose that the air temperature on a given day is 38 oC and that the water vapor pressure is 15.5 mm of mercury. The equilibrium vapor pressure at 3OoC (as given in Appendix Bi Table 12) is 31.8 mm of mercury. The relative humidity is
15.5 mm / 31.8mm x 100% = 48.7%
Relative humidity depends on both the temperature and the amount of water vapor in the atmosphere. The temperature at which a given amount of water vapor will exert equilibrium vapor pressure is called the dew point.
In the above example, the dew point is 18 oC because at that temperature the equilibrium vapor pressure of water is 15.5 mm of mercury.
7.22 Boiling When water is heated sufficiently its vapor pressure will eventually equal the combined pressure of the atmosphere and the liquid pressure of the water. Vaporization then occurs at such a rapid rate throughout the water that the water becomes agitated. Rapid vaporization that occurs when the vapor pressure of the liquid equals the pressure on its surface is called boiling.
If the pressure on the liquid surface is one atmosphere (760 mm of mercury), the temperature at which boiling occurs is called the normal boiling point. If the pressure on the liquid surface is greater than one atmosphere, boiling occurs at a higher temperature than the normal boiling point; if the pressure on the liquid surface is less than one atmosphere, boiling occurs at a lower temperature than the normal boiling point.
Greater pressure is largely the result of the greater number of collisions of the particles against the surface of the liquid. Consequently, the kinetic energy of the liquid, and thus its temperature, must be raised to make boiling possible. The opposite is true when the pressure on a liquid is decreased.
Water or any other liquid that is boiling rapidly does not get hotter than when it is boiling slowly. While a liquid is boiling away, the boiling temperature remains constant until all the liquid has been vaporized.
Solids or gases dissolved in a liquid change the liquid's boiling temperature. For example, salt water boils at a higher temperature than pure water. In general, so dissolved in liquids raise the boiling temperature gases dissolved in liquids lower the boiling temperature.
7.23 Plasma A gas that is capable of conducting electricity is called a plasma. Gases do not ordinarily conduct electricity, but when they are heated to high temperature gas molecules collide vigorously with each other to form electrically charged particles called ions. These ions give the plasma the ability to conduct an electric current.
Plasma is sometimes called the fourth phase of matter. Under normal conditions, matter exists only as a solid, liquid, or gas. But at the high temperatures that prevail in sun and other stars, matter exists almost entirely in plasma phase. Because there are so many stars, it estimated that more than 99 percent of the matter in the universe is in the form of plasma.
SUMMARY
The smallest particle of any substance that is capable of stable, independent existence is a molecule. Molecules are composed of atoms. Atoms are the smallest particles of elements that can exist either alone or in combination with other atoms of the same or different elements.
The masses of individual atoms are usually expressed in atomic mass units, rather than kilograms. The atomic mass unit is based on the mass of the carbon-12 atom.
The kinetic theory of matter states that molecules of matter are in constant motion and undergo perfectly elastic collisions.
The phase of matter (solid, liquid, or gas) is determined by the forces acting between molecules and the energy the molecules possess.
Properties of solids such as diffusion, cohesion, adhesion, tensile strength, ductility, malleability, and elasticity depend on molecular forces and/or molecular motion. Hooke's law states that, within limits, strain (the deformation of a solid) is directly proportional to the stress producing the strain. The ratio of stress to strain is called Young's modulus.
Molecular forces, molecular motion, and the weight of molecules are several factors on which liquid properties such as diffusion, cohesion, adhesion, viscosity, surface tension, and capillarity all depend.
Melting is the change of phase from a solid to a liquid. Crystalline solids have characteristic melting points; noncrystalline solids do not have definite melting points. Most substances expand upon melting. Water is an important exception. Pressure and dissolved materials change the melting point and freezing point of substances.
Gases expand, exert pressure, and diffuse. Vaporization is the change of phase from a solid or liquid to a gas or vapor. The added pressure exerted by vapor molecules in equilibrium with liquid molecules is called equilibrium vapor pressure. Boiling is rapid vaporization that occurs when the vapor pressure of a liquid is equal to the pressure on its surface. In the stars, matter is in the form of plasma.
VOCABULARY
adhesion, atom, atomic mass unit, boiling point, Brownian movement, capillarity, cohesion, condensation, diffusion, ductility, elasticity, elastic limit, elongation, strain, equilibrium vapor pressure, evaporation, freezing point, Hooke's law, kinetic theory, malleability, mass number, melting point, meniscus, molecule, phase, plasma, regelation, shear strain strain, stress, sublimation, surface tension, tensile strength, vaporization, viscosity, volume strain, Young's modulus.
Ah Yaz Indeed!
 Assignment Sheet for this Research Text Only.
Assignment Sheet for this Research Text Only.
 Go to Textbook Assignments for Portfolio:
Go to Textbook Assignments for Portfolio:
.................................First Semester
.................................Second Semester