Heating and Chemical Effects

Heating and Chemical Effects

The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
HEATING AND CHEMICAL EFFECTS
HEATING EFFECTS
18.1 Energy of an electric Current The relationships among objects, time, and space are basic concerns of physics. Work, power, and energy are important physical concepts involved in these relationships. We have described energy as the ability to do work and measured it in units of work and heat.
To maintain an electric current, energy must be supplied by the source of emf. Chemical energy, which can be thought of as the potential energy of chemical bonds, is transformed into electric energy in an electrochemical cell. Thus a charge acquires energy within the cell or other source of emf and expends this energy in the electric circuit.
In Section 16.10 we recognized that one joule of work is done when one coulomb of charge is moved through a potential difference of one volt.
W = qV
One coulomb of charge transferred in one second constitutes one ampere of current.
I = q/t and q = It
Therefore
W = Vlt (Equation 1)
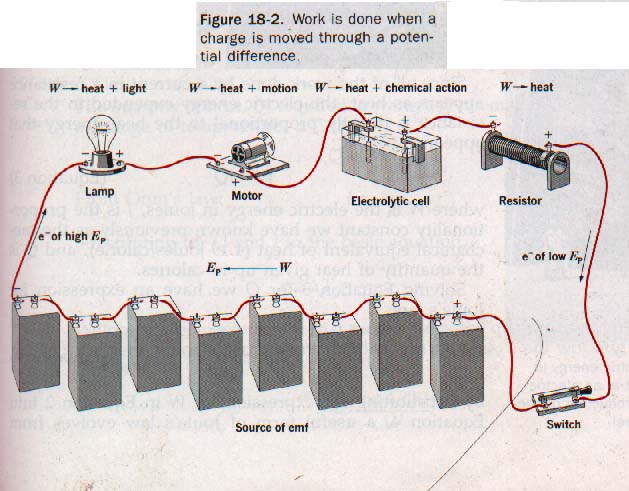
Within a source of emf, the charge is moved in opposition to the potential difference, and work, W, is done on electrons by the source of emf. As the charge moves through the external circuit in response to the potential difference across the circuit, work, W, is done by the electrons on the components of the circuit. It is this electric energy expended in the external circuit, that is available perform useful work.
We can observe the work done by an electric current in a variety of ways. If a lamp is in the circuit, part of the work appears as light; if an electric motor is in the circuit, part of work appears as rotary motion; if an electroplating cell in the circuit, part of the work promotes chemical action the cell.
In all cases, part of the work, W, appears as heat because of the inherent resistance of the circuit components. If an ordinary resistor comprises the circuit, all the goes to the production of heat since no mechanical or chemical work is done. See Figure 18-2.
18.2 Energy Conversion in Resistance Suppose short of No. 30 gauge copper wire and German silver are connected in series and placed in the circuit shown in Figure 18-3.
When the switch is closed, the German silver wire may become red hot and melt. The copper will become warm but is less likely to melt. The melting point of German silver is about 30 Co higher than that copper, but its resistivity is nearly 20 times higher than of copper. Since the two wires were in series, the same magnitude of current was in each. We may infer that a greater quantity of electric energy was converted to heat in the wire having the higher resistance.
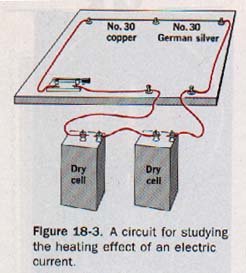
We may infer that a greater quantity of electric energy was converted to heat in the wire having the higher resistance. And the heat is greater in a wire having the greater current. So
W = I2Rt
Where W is the work in joules, I is the current in amperes, R is the resistance in ohms, and t is the time in seconds.
18.3 Joule's Law James Prescott Joule found that the heat developed in a conductor is directly proportional to the resistance, the square of the current, and the time that the current is maintained.
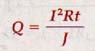

ELECTROLYSIS
18.7 Electrolytic Cells The close relationship between chemical energy and electric energy has already been discussed. Spontaneous reactions that involve the transfer electrons from one reactant to the other were described in Section 17.3. Such reactions can be sources of emf.
In electrochemical cell chemical energy is converted to electric energy as the spontaneous electron transfer reaction proceeds. The products of the reaction have less energy than did the reactants.
Similar reactions that are not spontaneous can be forced occur if electric energy is supplied from an external source as in charging a storage battery. In such force directions the products have more chemical energy than did the reactants. This means electric energy from the external of emf is transformed into chemical energy as reaction the proceeds.
An arrangement in which a forced electrons transfer reaction occurs is known as an electrolytic cell. The basic requirements for an electrolytic cell are shown in Figure 18-6.
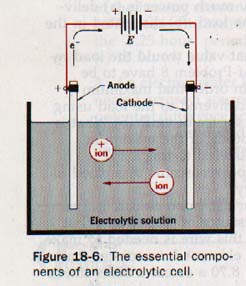
The conducting solution contains an electrolyte that furnishes positively and negative ions. Two electrodes are immersed in the electrolyte. The negative terminal of a battery or other direct current source is connected to one electrode to cathode of the cell, the positive terminal of the connected to the other electrode to form the anode of the cell.
When the circuit is closed, the cathode becomes negatively charged and the anode positively charged. Positive ions migrate to the cathode where they acquire electrons of high potential energy and are then discharged. Negatively charged ions migrate to the anode and are discharged by giving up electrons of low potential energy to the anode.
The end result of electrolysis depends on the nature of the electrolyte, the kinds of electrodes, and to some extent the emf of the source. Electrolytic cells are useful in decomposing compounds, plating metals, and refining metals.
18.8 Electroplating Metals An electrolytic cell is set up with a metallic copper anode and positively charged copper(II) ions, Cu++, in solution. When a small potential difference is placed across the cell, the Cu++ ions acquire electrons from the cathode and form copper atoms. The following chemical equation represents this reaction.
Cu++ + 2e- ---> CuO (Cathode reaction)
These copper atoms plate out on the cathode surface. Simultaneously, atoms of the copper anode give up electrons to this positively charged anode and form Cu++ ions that go into the solution to replace those being plated as copper atoms. The chemical reaction is
Cuo ---> Cu++ + 2e- (Anode reaction)
These Cu++ ions pass into the solution from the anode at same rate as similar Cu++ ions leave the solution and the plate on the cathode. To maintain the concentration of Cu++ ions in the electrolytic solution, Cu++ ions continuously pass into the solution from the anode. result is that the anode is used up in the plating process simplified diagram of a copper-plating cell is in Figure 18-7.
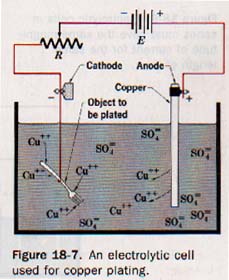
The reaction at the cathode makes an electrolytic cell for the electrolytic deposition, or electroplating, of one metal on the surface of another. The object to be plated is used as the cathode of the cell. The plating metal is used as the anode. The conducting solution contains a salt of the metal to be plated out onto the cathode.
18.9 Faraday's Laws of Electrolysis Faraday discovered that a current that deposited a half-penny-weight of silver in ten minutes would deposit one penny-weight of silver in twenty minutes. This discovery suggests an important relationship between the quantity of electric charge that passes through an electrolytic cell and the quantity of a substance liberated by the chemical action.
Faraday's First Law: the mass of an element deposited or liberated during electrolysis is proportional to the quantity of charge that passes.
Q = It
The electrochemical equivalent, z, of an element is the mass in grams of the element deposited or liberated by 1 coulomb of electric charge.
1 faraday = 96,500 coulombs
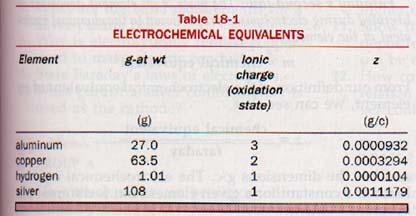
Faraday's Second Law: The mass of an element deposted or liberated during electrolysis is proportional to the chemical equivalent of the element.
So we use the formula:
m = zIt
where m is the mass in grams of an element deposited or liberated, z, is the electrochemical equivalent in g/c of the element, I is the current in amperes, and t is the time in seconds.
SUMMARY
The electric energy expended in a resistance is converted to heat. The relationship among the heat developed in a circuit element.
Electric power dissipated in the resistance of a circuit is proportional to the square of the circuit current. Power dissipated in the internal resistance of the source of emf is wasted.
When it is important to deliver maximum power to the load, the load resistance and that of the source of emf must be matched. Electrolytic cells are driven by an external source of emf. Electric energy is then transformed to chemical energy through electron-transfer reactions at the cell electrodes. The products have higher chemical energy than the reactants.
Electrolytic cells are useful in decomposing compounds, plating metals, and refining metals .
Faraday's laws of electrolysis provide an insight into the quantitative significance of energy transformations in chemical reactions. These laws relate the mass of an element deposited during electrolysis to the quantity of charge that passes through the cell and to the chemical equivalent of the element.
VOCABULARY
anode, electrochemical equivalent, faraday, cathode, electrolysis, Faraday's first law, chemical equivalent, electrolyte, Faraday's second law, electrolytic cell, Joule's law, electrochemical cell, electroplating, spontaneous reaction.
Ah Yaz Indeed!
 Assignment Sheet for this Research Text Only.
Assignment Sheet for this Research Text Only.
 Go to Textbook Assignments for Portfolio:
Go to Textbook Assignments for Portfolio:
.................................First Semester
.................................Second Semester