Electrostatics

Electrostatics

The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
ELECTROSTATICS
Benjamin Franklin performed many experiments on the nature of electricity. In one of these, he flew a kite during a thunderstorm to show that clouds are electrically charged and that he could draw sparks from a key tied to the kite string-- at the risk of his life.
ELECTRIC CHARGE
16.1 Charges at Rest We can observe about us a number of physical effects that are sometimes produced by rubbing pieces of dry matter. Sometimes an annoying shock is felt when the door handle of an automobile (heh, heh) is touched after one slides over the plastic-covered seat.
We may feel a shock after we walk on a woolen carpet and then touch a doorknob (yeeeowwwie) or other metal object. The slight crackling sound heard when dry hair is brushed and the tendency of thin sheets of paper to resist separation are other common observations of these physical effects.
When an object shows effects of the type we have described, we say that it has an electric charge. The process that produces electric charges on an object is called electrification.
Electrification is most apparent when the air is dry. An object that is electrically charged can attract small bits of cork, paper, or other lightweight particles. Because the electric charge is confined to the object and is not moving, it is called an electrostatic charge. Thus static electricity is stationary electricity in the form of an electric charge at rest. Static electricity is commonly produced by friction between two surfaces in close contact.
16.2 Two Kinds of Charge We can detect the presence of an electrostatic charge by means of an instrument called an electroscope. The simplest kind of electroscope is a small ball of wood pith or styrofoam suspended by a silk thread. This electroscope is more sensitive if the pith ball is coated with aluminum or graphite. Such an instrument is shown in Figure 16-1.
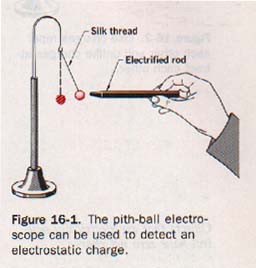
Suppose a hard rubber or plastic rod is charged by stroking it with flannel or fur (kitteeee). If the end of the charged rod is then held near a simple electroscope, the pith ball is attracted to the rod.
If the pith ball is allowed to come in contact with the charged rod, it immediately rebounds and is then repelled by the rod. We can reasonably assume that some of the charge has been transferred to the pith ball so that both the rod and the ball are now similarly charged.
Now suppose a glass rod is charged by stroking it with silk. If the glass rod is held near the charged electroscope, the pith ball is attracted rather than repelled as it was with the charged rubber rod. These effects of attraction and repulsion can be explained if we assume that there are two kinds of electric charge.
The electric charge produced on the rubber rod when the rod is stroked with flannel or fur is called a negative charge. The rod is said to be charged negatively.
The electric charge produced on the glass rod when the rod is stroked with silk is called a positive charge. The rod is said to be charged positively.
From a study of atomic structure it is known that all matter contains both positive and negative charges. For simplification, however, the diagrams that follow show only the excess charge. If an object has an excess negative charge, the net charge will be indicated by negative (-) signs. If it has an excess positive charge, the net charge will be indicated by positive (+) signs. A neutral object will have no sign because it has the same amount of each kind of charge and therefore a net charge of zero.
Two pith balls that are negatively charged by contact with a charged rubber rod repel each other. Similarly, two pith balls that are positively charged by contact with a charged glass rod also repel each other. However, if a negatively charged pith ball is brought near a positively charged pith ball, they attract each other. See Figure 16-2. These observations are summarized in a basic law of electrostatics:
Objects that are similarly charged repel each other; objects that are oppositely charged attract each other.

16.3 Electricity and Matter As an understanding of the nature of static electricity requires a knowledge of the basic concepts regarding the structure of matter, we will briefly discuss these concepts now. (These concepts will be discussed more fully in Chapter 23.) All matter is composed of atoms, of which there are many different kinds.
Each atom consists of a positively charged nucleus surrounded by negatively charged electrons.
Protons and neutrons are tightly packed into the very dense nucleus. Because each proton possesses a single unit of positive electric charge and neutrons, as their name suggests, are neutral particles, the nucleus is positively charged. This charge is determined by the number of protons the nucleus contains.
All electrons surrounding the nucleus are alike. They all carry the same amount of negative electric charge-- one unit of negative charge per electron. Because an atom is electrically neutral, we know that the nucleus has a positive charge equal in magnitude to the total negative electronic charge. Thus the number of electrons of a neutral atom equals the number of protons.
The rest mass of an electron is 9.1095 x 10-31 kg. The mass of a proton is 1.6726 x 10-27 kg and that of a neutron is 1.6750 x 10-27 kg. Both the proton and the neutron have masses that are nearly 2000 times greater than the mass of an electron. The mass of an atom is almost entirely concentrated in the nucleus.
Protons and neutrons are bound together within the nucleus by strong forces acting through very short distances. By comparison, the repulsions between protons that are due to their similarity of charge are weak forces.
The electrons are retained in the atom structure by the electric attraction exerted by the positive nuclear charge. In general the outermost electrons of higher energies are held less firmly in the atom structure than inner electrons of lower energies. The outer electrons of the atoms of metallic elements in particular are loosely held and are easily influenced by outside forces.
When two appropriate materials are in close contact, some of the loosely held electrons can be transferred from one material to the other.
If a hard rubber rod is stroked with fur, some electrons can be transferred from the fur to the rod. The rubber rod becomes negatively charged because it has a net excess of electrons, and the fur becomes positively charged because it has a net deficiency of electrons.
Similarly, when a glass rod is stroked with silk, some electrons are transferred from the glass to the silk. The glass is positively charged because it has a net deficiency of electrons, and the silk is negatively charged because it has a net excess of electrons. All these charged states result from the transfer of electrons.
Electric charge is a scalar quantity. The net charge on an object is the sum of its positive charges minus the sum of its negative charges.
16.4 The Electroscope Two common types of electroscopes that are more sensitive than the simple pith ball device are shown in Figure 16-3.
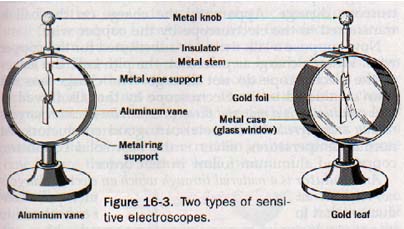
The vane electroscope consists of a light aluminum rod mounted by means of a central bearing on a metal support that is insulated from its metal stand. When charged, the vane is deflected at an angle by electrostatic repulsion. The angle of deflection depends on the magnitude of the charge.
The leaf electroscope consists of very fragile strips of gold leaf suspended from a metal stem that is capped with a metal knob. The leaves are enclosed in a metal case with glass windows for their protection, and the metal stem is insulated from the case.
When electrified, the leaves diverge because of the force of repulsion due to their similar charge. Good sensitivity is realized because of the very low mass of the gold leaf.
16.5 Conductors and Insulators Suppose that an aluminum coated pith ball is suspended by a silk thread and the ball is connected to the knob of a leaf electroscope by means of a copper wire, as shown in Figure 16-4.
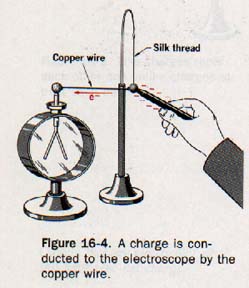
When a charge is placed on the pith ball, the leaves of the electroscope diverge. Apparently the charge on the ball is transferred to the electroscope by the copper wire.
Now suppose a silk thread is substituted for the copper wire. When a charged is placed on the pith ball, the leaves of the electroscope do not diverge. The charge has not been conducted to the electroscope by the silk thread.
A conductor is a material through which an electric charge is readily transferred. Most metals are good conductors. At normal temperatures, silver is the best solid conductor; copper and aluminum follow in that order.
An insulator is a material through which an electric charge is not readily transferred. Good insulators are such poor conductors that for practical purposes they are considered to be nonconductors. Glass, mica, paraffin, hard rubber, sulfur, silk, dry air, and many plastics are good insulators.
Liquid solutions and confined gases conduct electricity in a different way than solids. At this time we are interested in the conductivity of solids. We can use our knowledge of the structure of matter in describing why some materials are conductors and why some are insulators.
A few grams of matter contain a very large number of atoms and, except for hydrogen, an even larger number of electrons. For example, 27 g of aluminum consists of 6.02 x 1023 aluminum atoms ( one mole) containing 7.83 x1024 electrons.
On an atomic scale, there is one electron in approximately 2.2 atomic mass units of matter. (One atomic mass unit = 1.66 x 10-24 g of matter.) Metals have close-packed crystal structures. The crystal lattice cansists of positively charged particles permeated by a cloud of free electrons. This cloud of free electrons is commomly referred to as the eIectron gas. The binding force in such structures is the attraction between the positively charged metal ions and the electron gas.
The loosely held outer most electrons of the metal atoms have been "donated" to the electron gas and belong to the crystal as a whole. These electrons are free to migrate throughout the crystal lattice. Their migration gives rise to the high electric conductivity commonly associated with metals.
A good conductor contains a large number of free electrons whose motions are relatively unimpeded within the material. Since like charges repel, the free electrons spread throughout the material in order to relieve any local concentration of charge. If such a material is in contact with a charged body, the free electrons surge in a common direction. If the charged object is deficient in electrons (positively charged), this surge is in the direction of the object.
If the charged object has an excess of electrons (negatively charged), the surge is away from the object. See Figure 16-5. In either case a transfer of electric charge continues until the repulsive forces between the free electrons are in equilibrium throughout the entire system.
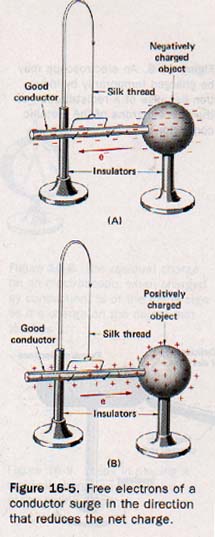
An insulator is characterized by a lack of free electrons because even the outermost electrons are rather firmly held within the atom structure. Thus the transfer of charge through an insulator is usually negligible. If an excess of electrons is transferred to any particular region of such a material, the extra electrons remain in that region for some time before they gradually leak away.
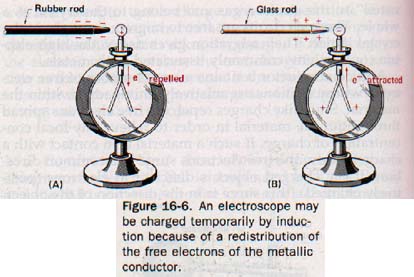
16.6 Transferring EIectrostatic Charges Suppose a rubber rod is charged negatively by stroking it with kitteeee. If the rod is brought near the knob of an electroscope, the leaves diverge. If the rod is removed, the leaves collapse; no charge remains on the electroscope.
The charge that makes the leaves diverge is called an induced charge. The electroscope is said to be charged temporarily by induction.
We can reason that the negative charge on the rod when brought near the metal knob of the electroscope repels free electrons in the knob and metal stem and forces them down to the leaves. See Figure 16-6(A).
The force of repulsion of the extra electrons on the leaves causes them to diverge. The knob is then deficient in electrons and is positively charged. As soon as the force of repulsion exerted by the charged rod is withdrawn, the excess free electrons on the leaves scatter throughout the stem and knob, restoring the normal uncharged state throughout the electroscope.
Similarly, a glass rod that has been stroked with silk temporarily induces a positive charge on the leaves of an electroscope by attracting electrons up through the stem to the knob. See Figure 16-6(B).
Electrostatic experiments are best performed in dry air.
In moist air an invisible film of water condenses on the surfaces of objects, including those of charged insulators. Dissolved impurities in the film make these surfaces conductive, and an isolated charge cannot be maintained for any length of time.
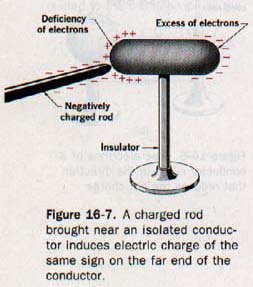
Any conducting object when properly isolated in space can be temporarily charged by induction. The region of the object nearest the charged body will acquire a charge of the opposite sign; the region farthest from the charged body will acquire a charge of the same sign. See Figure 16-7. This statement is consistent with the basic law of electrostatics stated in Section 16.2.
A charge can be transferred by conduction. If we touch the knob of an electroscope with a negatively charged rubber rod, the leaves diverge. When the rod is removed, the leaves remain apart, indicating that the electroscope retains the charge. How can we determine the na
We can reason that some of the excess electrons on the rod have been repelled onto the knob of the electroscope. This would be true only for the region of the rod immediately in contact with the electroscope since rubber is a very poor conductor and excess electrons do not migrate freely through it. Any free electrons thus transferred to the electroscope, together with other free electrons of the electroscope itself, would be repelled to the leaves by the excess electrons remaining on the parts of the rod not in contact with the electroscope.
When the rod is removed, and with it the force of repulsion, the electroscope is left with a residual negative charge of a somewhat lower density. This deduction can be verified by bringing a positively charged glass rod near the electroscope to induce a positive charge on the leaves. The leaves collapse and diverge again when the glass rod is removed. See Figure 16-8.
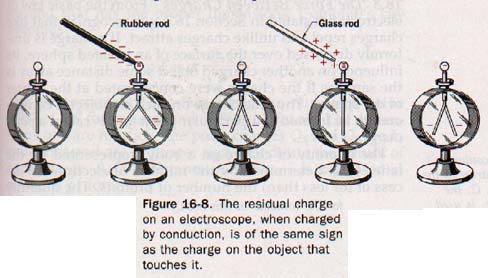
Any conducting object, properly isolated in space and charged by conduction, acquires a residual charge af the same sign as that of the body touching it. An electroscope with a known residual charge can be used to identify the nature of the charge on another object. This second object merely needs to be brought near the knob of this charged electroscope.
16.7 Residual Charge by Induction When a charged rubber rod is held near the knob of an electroscope, there is no transfer of electrons between the rod and the electroscope. If a path is provided for electrons to be repelled from the electroscope while the repelling force is present, free electrons escape. Then if the escape path is removed before removing the repelling force, the electroscope is left with a deficiency of electrons, giving it a residual positive charge.
We can verify this conclusion by bringing a positively charged glass rod near the knob of the charged electroscope. The leaves of the electroscope diverge even more. When the charged rod is withdrawn, the leaves fall back to their original divergence and remain apart. The steps in placing a residual charge on an electroscope by induction are shown in Figure 16-9.

Similarly, we can induce a residual negative charge an electroscope using a positively charged glass rod. An isolated conductor is given a residual charge by induction, charge is opposite in sign to that of the object inducing it.
16.8 The Force Between Charges From the basic law electrostatics stated in Section 16.2, we recognize that like charges repel and unlike charges attract. If a charge is uniformly dispersed over the surface of an isolated sphere, influence on another charged object some distance away the same as if the charge were concentrated at the center of the sphere.
Thus the charge on such an object is considered to be located at a particular point and is called a point charge.
The quantity of charge on a body, represented by the letter Q, is determined by the number of electrons in excess of (or less than) the number of protons. The quantity of charge is measured in coulombs (c), named for the French physicist Charles Augustin de Coulomb (1736-1806).
1 coulomb = 6.25 x lOl8 electrons
Thus the charge on one electron, expressed in coulombs, is the reciprocal of this number and the sign of Q is -.
e- = 1.60 x 10-19 c
Similarly, the charge on one proton is 1.60 x 10-19 coulomb and the sign of Q is +.
The coulomb is a very large unit of charge for the study of electrostatics. Frequently it is convenient to work with a fraction of this unit called the microcoulomb.
Coulomb's many experiments with charged bodies led him to conclude that the forces of electrostatic attraction and repulsion obey a law similar to Newton's law of universal gravitation.
We now recognize his conclusions as Coulomb's Law of electrostatics: The force between two point charges is directly proportional to the product of their magnitudes and inversely proportional to the square of the distance between them.
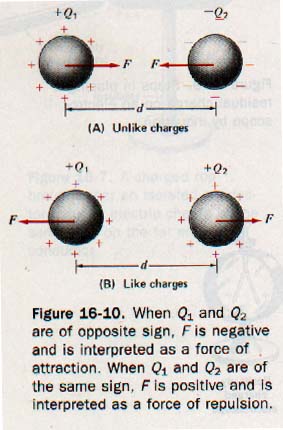
Charged bodies approximate point charges if they are small compared to the distances separating them. See Figure 16-10.
Coulomb's law can be expressed as
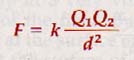
The proportionality constant k has the numerical value 8.987 x 109 for vacuum and 8.93 x 109 for air. The dimensions of k are n m2/c2. The point charges, Q1 and Q2 are in coulombs and are of proper sign to indicate the nature of each charge. As d is distance in meters, F is expressed in newtons of force.
In Figure 16-10(A) (above) the charges Q1 and Q2 have opposite signs and the force F acts on each charge to move it toward the other. In Figure 16-10(B) (above), charges Q1 and Q2 have the same sign and the force F acts on each charge to move it away from the other. The force between the two charges is a vector quantity that acts on each charge.
16.9 Electric Fields The concept of a field of force will be helpful as we consider the region surrounding an electrically charged body. A second charge brought into this region experiences a force according to Coulomb's law. Such a region is an electric field. An electric field is said to exist in a region of space if an electric charge placed in that region is subject to an electric force.
Let us consider a positively charged sphere +Q of Figure 16-11(A) isolated in spare. A small positive charge +Q, which we shall call a test charge, is brought near the surface of the sphere.
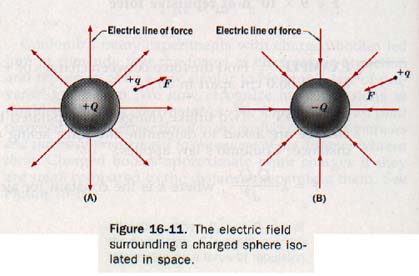
Since the test charge is in the electric field of the charged sphere and the charges are similar, it experiences a repulsive force directed radially away from +q. Were the charge on the sphere negative, as in Figure 16I1(B), the force acting on the test charge would be directed radially toward -q.
An electric line of force is a line so drawn that a tangent to it at any point indicates the orientation of the electric field at that point. We can imagine a line of force as the path of a test charge moving slowly in a very viscous medium in response to the force of the field. By convention, electric lines of force originate at the surface of a positively charged body and terminate at the surface of a negatively charged body, each line of force showing the direction in which a positive test charge would be accelerated in that part of the field.
A line of force is normal to the surface of the charged body where it joins that surface.
The intensity, or strength, of an electrostatic field, as well as its direction, can be represented graphically by lines of force.
The electric field intensity is proportional to the number of lines of force per unit area normal to the field. Where the intensity is high, the lines of force will be close together. Where the intensity is low, the lines of force will be more widely separated in the graphical representation of the field.
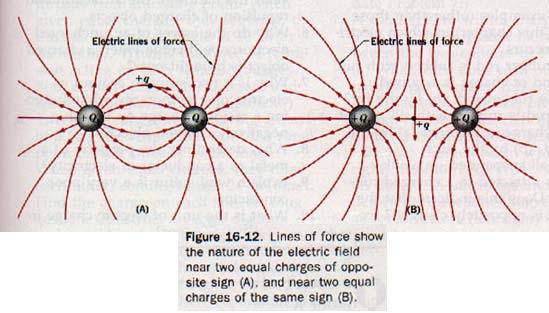
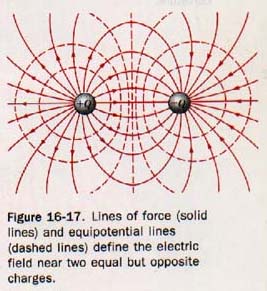
In Figure 16-12(A), electric lines of force are used to show the electric field near two equally but oppositely charged objects. At any point in this field the resultant force acting on a test charge +q can be represented by a vector drawn tangent to the line of force at that point. The electric field near two objects of equal charge of the same sign is shown by the lines of force in Figure 16-12(B). The resultant force acting on a test charge +q placed at the midpoint between these two similar charges would be zero.
POTENTIAL DIFFERENCE
16.10 Electric Potential Let us consider the work done by gravity on a wagon coasting down a hill. The wagon is within the gravitational field of the earth and experiences a gravitational force causing it to travel downhill. Work is done by the gravitational field, so the energy expended comes from within the gravitational system. The wagon has less potential energy at the bottom of the hill than it had at the top; in order to return the wagon to the top, work must be done on it.
However, in this instance, the energy must be supplied from an outside source to pull against the gravitational force. The energy expended is stored in the system, imparting to the wagon more potential energy at the top of the hill than it had at the bottom.
Similarly, a charge in an electric field experiences an electric force according to Coulomb's law. If the charge moves in response to this force, work is done by the electric field. Energy is removed from the system. If the charge is moved against the coulomb force of the electric field, work is done on it using energy from some outside source, this energy being stored in the system.
If work is done as a charge moves from one point to another in an electric field or if work is required to move a charge from one point to another, these two points are said to differ in electric potential. The magnitude of the work is a measure of this difference of potential.
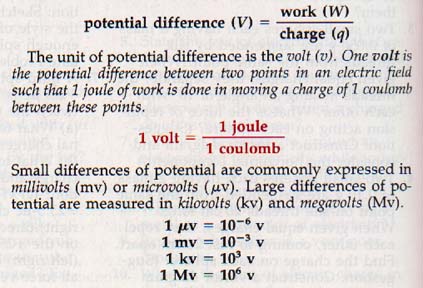
The concept of potential difference is very important in the understanding of electric phenomena. The potential difference, V, between two points in an electric field is the work done per unit charge as a charge is moved between these points.
The unit of potential difference is the volt (v). One volt is the potential difference between two points in an electric field such that 1 joule of work is done in moving a charge of 1 coulomb between these points.
16.11 Distribution of Charges Michael Faraday performed several experiments to demonstrate the distribution of charge on an isolated object. He charged a conical silk bag like that shown in Figure 16-14 and found that the charge was on the outside of the bag.
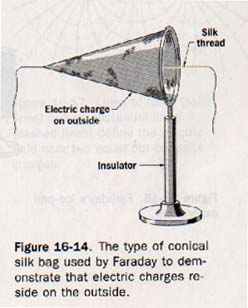
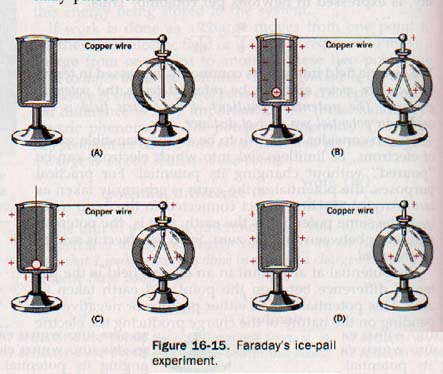
By pulling on the silk thread, he turned the bag inside out and found the charge was again on the outside. The inside of the showed no electric charge in either position. Faraday connected the outer surface of an insulated metal pail to an electroscope by means of a wire, as shown in Figure 16-15(A). He then lowered alternately charged metal ball supported by a silk thread into the pail. The leaves of the electroscope diverged (B), indicating a charge by induction.
The positive charge on ball attracted free electrons in the pail to the inner surface, leaving the outside of the pail and the electroscope positively charged. When the ball was allowed to come in contact with the inside of the pail (C) and was then removed the leaves remained apart without change.
It was found that the ball no longer had a charge after its removal. The fact that the electroscope remained unchanged after the ball was removed (D) indicated (1) that there was no redistribution of positive charge on the outside surface of the pail and (2) that the outside of the pail (and the electroscope) had acquired a net charge equal to the charge originally placed on the ball.
From these and other experiments with isolated conductors we can conclude that:
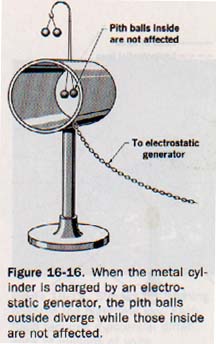
1. All the static charge on a conductor lies on its surface. Electrostatic charges are at rest. If the charge were beneath the surface so that an electric field existed within a conductor, free electrons would be acted upon by the coulomb force of this field. Work would be done, the electrons would move because of a difference of potential, and energy would be given up by the field. Since movement is not consistent with a static charge, the charge must be on the surface and the electric field must exist only externally to the surface of a conductor. See Figure 16-16.
2. There can be no potential difference between two points on the surface of a charged conductor. A difference of potential is a measure of the work done in moving a charge from one point to another. As no electric field exists within the conductor, no work is done in moving a charge between two points on the same conductor. No difference of potential can exist between such points.
3. The surface of a conductor is an equipotential surface. All points on a conductor are at the same potential and no work is done by the electric field in moving a charge residing on a conductor. If points of equal potential in an electric field near a charged object are joined, an equipotential line or surface within the field is indicated. No work is done when a test charge is moved in an electric field along an equipotential surface.
4. Electric lines of force are normal to equipotential surfaces. A line of force shows the direction of the force acting on a test charge in an electric field. It can be shown that there is no force acting normal to this direction. Thus no work is done when a test charge is moved in an electric field normal to the lines of force. See Figure 16-17.
5. Lines of force originate or terminate normal to the conductive surface of a charged object. Since the surface of a conductor is an equipotential surface, lines of force must start out perpendicularly from the surface. For the same reason, a line of force cannot originate and terminate on the same conductor.
16.12 Effect of the Shape of a Conductor A charged spherical conductor perfectly isolated in space has a uniform charge density, or charge per unit area, over the outer surface. Lines of force extend radially from the surface in all directions and the equipotential surfaces of the electric field are spherical and concentric. Such symmetry is not found in all cases of charged conductors. A charge acquired by a nonconductor such as glass is confined to its original region until it gradually leaks away. The charge placed on an isolated metal sphere quickly spreads uniformly over the entire surface. If the conductor is not spherical, the charge distributes itself cording to the surface curvature, concentrating around points.
The pear-shaped conductor illustrated in Figure 16-18 shows the charge more concentrated on curved regions and less concentrated on the straight regions. If the small end is made more pointed, the density will increase at that end.

16.33 Discharging Effect of Points In Figure 16-18 lines of force and equipotential lines are shown more concentrated at the small end of the charged conductor. Geometry indicates that the intensity of the electric or potential gradient, in this region is greater than where around the conductor.
If this surface is reshaped a sharply pointed end, the field intensity can become great enough to cause the gas molecules in the air surrounding the pointed end to ionize. Ionized air consists of gas molecules from which an outer electron has been removed thus allowing both the positively charged ion and the freed electron to respond to the electric force.
When the air is ionized, the point of the conductor is rapidly discharged. There are always a few positive ions and free electrons present in the air. The intense electric field near a sharp point of a charged conductor will set these charged particles in motion such that the electrons are driven in one direction and the positive ions in the opposite direction. Violent collisions with other gas molecules will knock out some electrons and produce more charged particles.
In this way air can be ionized quickly when it is subjected to a sufficiently large electric stress.
In dry air at atmospheric pressure, a potential gradient of 30 kv/cm between two charged surfaces is required to ionize the intervening column of air. When such an air gap is ionized, a spark discharge occurs. There is a rush of free electrons and ionized molecules across the ionized gap, discharging the surfaces and producing heat, light, and sound. Big lightning!
Usually the quantity of static electricity involved is quite small and the time duration of the spark discharge is very short. Atmospheric lightning, however, is a spark discharge in which the quantity of charge is great. The intensity of an electric field near a charged object can be sufficient to produce ionization at sharp projections or sharp corners of the object. A slow leakage of charge can occur at these locations producing a brush or corona discharge. A faint violet glow is sometimes emitted by the ionized gases of the air.
A glow discharge known as St. Elmo's fire can sometimes be observed at night at the tips of ship masts and at the trailing edges of wing and tail surfaces of aircraft. The escape of charges from sharply pointed conductors is important in the operation of electrostatic generators and in the design of lightning arresters.
Lightning is a gigantic electric discharge in which electric charges rush to meet their opposites. The interchange can occur between clouds or between a cloud and the earth. See Figure 16-19.

According to the Lightning Protection Institute, one hundred bolts of lightning strike the earth every second, each bolt initiated by a potential difference of millions of volts.
There are no known ways of preventing lightning. However, there are effective means of protection from its destructiveness. Lightning rods, invented by Benjamin Franklin, are often used to protect buildings made of nonconducting materials from lightning damage.
Sharply pointed rods are strategically located above the highest projections of the building, and by their discharging effect, they normally prevent the accumulation of a dangerous electrostatic charge. Lightning rods are thoroughly grounded. If lightning strikes, the rods provide a good conducting path into the ground. Being well grounded, the steel frames of large buildings offer excellent protection from lightning damage.
Television receiving antennas, even though equipped with lightning arresters, do not protect a building from lightning. Without lightning arresters they are a distinct hazard because they are not grounded.
26.44 Capacitors Any isolated conductor is able to retain an electrostatic charge to some extent. If we place a positive charge on such a conductor by removing electrons, the potential is raised to some positive value with respect to ground. Conversely, a negative charge placed on the conductor results in a negative potential with respect to ground.
By increasing the charge, we increase the potential of the conductor since the potential of an isolated conductor is a measure of the work done in placing a charge on the conductor. It is evident that we can continue to increase the charge until the potential with respect to ground or other conducting surface becomes so high that corona or spark discharges occur.
However, if the conductor is in an evacuated space, it could be raised to a much higher potential by continuing the addition of charge. Suppose a charged conductor is connected to an electroscope, as shown in Figure 16-20(A). The leaves of the electroscope will diverge indicating the potential of the charged conductor.
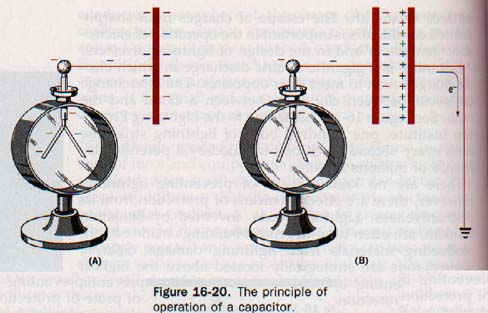
The electroscope is now a part of the conducting surface and there can be no difference of potential between different regions of a single charged ducting surface.
Now suppose a grounded conductor is brought near charged conductor, as shown in Figure 16-20(B). A negative charge is induced on this second conductor as electrons are repelled to ground by the negative field the first conductor. The leaves of the electroscope collapse. The closer we move the grounded plate to charged conductor, the more pronounced is this effect.
Because of the attractive force of the induced charge on grounded plate, less work is required to place the negative charge on the first conductor. Its potential is sequentially reduced accordingly. A greater charge can be placed on this conductor to raise the potential back the initial value.
A combination of conducting plates separated by an insulator that is used to store an electric charge is known as a capacitor. The area of the plates, their distance of separation, and the character of the insulating material separating them determine the charge that can be placed on a capacitor.
The larger the charge, the greater is the potential between the plates of a capacitor. The ratio of charge Q to the potential difference V is a constant for a given capacitor and is known as its capacitance, C.
Capacitance is the ratio the charge on either plate of a capacitor to the potential between the plates. Thus
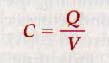
where C is the capacitance of a capacitor, Q is the quantity of charge on either plate, and V is the potential difference between the conduction plates.


16.15 Dielectric Materials Faraday investigated the effects of different insulating materials between the plates of capacitors. He constructed two capacitors with equal plate areas and equal plate spacing. Using air at normal pressure in the space between the plates of one and an insulating material between the plates of the other, he charged both to the same potential difference.
These capacitors are shown as C1 and C2 respectively in Figure 16-22. Faraday measured the quantity of charge on each capacitor and found that C2 had a greater charge than C1 by a factor K.
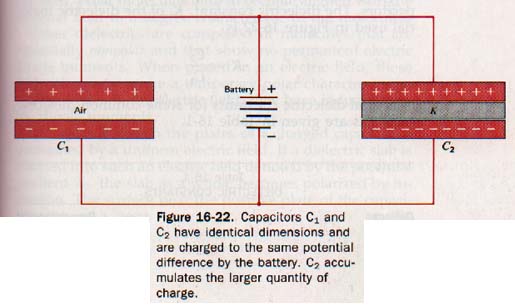
Q2 = KQ1
Except for the material separating the plates, the two capacitors are identical and have the same potential difference across their plates. The factor K by which the charge on C2 exceeds the charge on C1 must be due to a property of the insulating material of C2. The ratio Q2/V is larger than Q1/V by this factor K.
Many materials such as mica, paraffin, oil, waxed paper, glass, plastics, and ceramics can be used instead of air in the space between the plates of a capacitor. For each material, the resulting capacitance Q/V will have a different value.
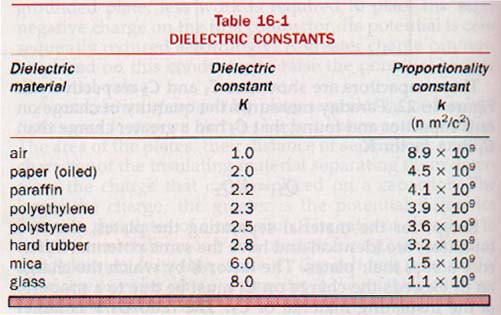
Materials used to separate the plates of capacitors are known as dielectrics. The ratio of the capacitance with a particular material separating the plates of a capacitor to the capacitance with a vacuum between the plates is called the dielectric constant, K, of the material.
Dry air at atmospheric pressure has a dielectric constant of 1.0006. In practice this is taken as unity (the same as vacuum) for dielectric constant determinations.
Dielectric constants are dimensionless numbers ranging from 1 to 10 for materials commonly used in capacitors. The dielectric constant, K, of the dielectric material used in Figure 16-22 is
K = C2/C1
Typical dielectric constants for some common dielectric materials are given in Table 16-1.
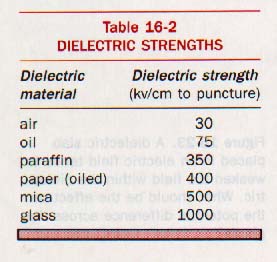
The puncture voltage is the voltage needed to break through the dielectric. That is to blast a hole right through the darn thing! Thus wiping out a capacitor. Here is a table of puncture voltages. Note that air breaks down at 30,000 volts/cm.
16.17 Combinations of Capacitors Suppose three capacitors of capacitances C1, C2 and C3 are connected in parallel, that is, with one plate of each capacitor connected to one conductor while the other plate is connected to a second conductor. See Figure 16-24(A).
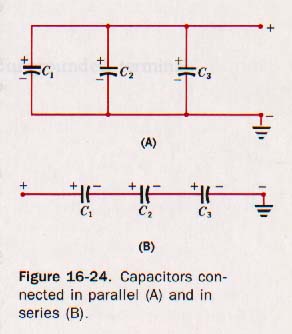
The plates connected to the + conductor are parts of one conducting surface. Those connected to the - conductor form the other conducting surface. If the three capacitors are charged, it is apparent that they must have the same difference of potential, V, across them. The quantity of charge on each must be respectively
CT = C1 + C2 + C3
For capacitors connected in parallel, the total capacitance is the sum of all the separate capacitances.
Now suppose we connect the three capacitors in series, as shown in Figure 16-24(B) above. A positive charge placed on C1 from the + source induces a negative charge on the second plate as the electrons are attracted way from the plate C2, which is connected to C1. A positive charge of the same magnitude is left on the + plate of C2. Similarly the plates of C3 acquire the same magnitude of charge. Thus
Q = Q1 = Q2 = Q3
The negative plate of C1 must be at the same potential with respect to ground as the positive plate of C2 since they are connected and are parts of the same conducting surface. Similarly the negative plate of C2 must be at the same potential as the positive plate of C3. The total difference of potential, must be equal to the sum of the separate potential differences across each capacitor.
For capacitors connected in series, the reciprocal of the total capacitance is equal to the sum of the reciprocals of all the separate capacitances.
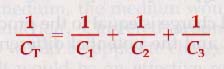
SUMMARY
Electric charges are of two kinds, negative and positive.
Like charges repel and unlike charges attract.
Electrification is the process that produces electric charges on an object. The process involves the transfer of free electrons.
An electroscope may be used to indicate the presence of an electric charge and to provide a rough measurement of its magnitude.
Substances are classified as conductors or insulators based on their ability to conduct an electric charge. Most metals are good conductors. Most nonmetals are good insulators because they are poor conductors of electric charges. The conduction of electric charge by solids is related to the availability of free electrons in their structures. Isolated bodies with conducting surfaces can be charged by either induction or conduction.
The force of attraction or repulsion between point charges is enunciated in terms of Coulomb's law. Charged bodies approximate point charges if they are small in comparison with the distance separating them. The region about a charged body in which the coulomb force exists is an electric field.
The path along which a positive test charge is driven by an electric field is called a line of force. Lines of force are used to represent both the direction and intensity of the field.
The difference in potential between two points in an electric field is defined in terms of work expended in moving a charge between two points and quantity of charge moved between these points.
Experiments with isolated conductors have shown that (1) the residual charge on a conductor resides on its surface; (2) there can be no difference of potential between two points on the surface of a charged conductor; (3) the surface of a conductor is an equipotential surface; (4) electric lines of force are normal to equipotential surfaces; and (5) lines of force originate and terminate normal to the conductive surface of a charged object.
Charge density on the conductive surface of a charged body varies with the surface curvature. The intensity of the electric field near sharp points may be great enough to ionize the surrounding air, thus discharging the body.
An arrangement of conducting surfaces separated by insulators and used to store electric charge is called a capacitor. The capacity for storing electric charge is its capacitance.
The dielectric constant of an insulating material is determined by comparing the material's effect on capacitance with the effect of air. Capacitors may be connected in series or in parallel. The total capacitance of the capacitor network is dependent upon the kind of connection used.
VOCABULARY
capacitance, capacitor, conductor (electric), corona discharge, coulomb, Coulomb's law of electrostatics, dielectric, dielectric constant, electric field, electric field intensity, electrification, electron gas, electroscope, farad, free electron, induced charge, insulator, line of force, microcoulomb, microfarad, picofarad, point charge, potential difference, potential gradient, residual charge, static charge, volt.
Ah Yaz Indeed!
 Assignment Sheet for this Research Text Only.
Assignment Sheet for this Research Text Only.
 Go to Textbook Assignments for Portfolio:
Go to Textbook Assignments for Portfolio:
.................................First Semester
.................................Second Semester