Physics: Science of Energy
An Introduction to Mighty Physics

Physics: Science of Energy
An Introduction to Mighty Physics

The purpose of this is to give quick reference to information or to use in an emergency (like if your text has accidentally been left under your desk at school).
This is NOT intended to replace reading the text with its excellent photographs, diagrams, charts, and tables.
SCIENCE IN TODAY'S WORLD
1.1 The Energy Problem Modern civilization would be impossible without the use of large quantities of energy. In the last forty years, energy usage in the United States increased more than 400% while the accompanying population increase has been only 72%. Thus the per capita use of energy in the United States has increased at a fast rate.
Until recently, the world's energy demands were met entirely through the use of fossil fuels: wood, coal, petroleum and natural gas. Since these are limited in quantity, however, other sources must be found.
Nuclear and solar energy are two alternate sources, and neither of them is limited. It has been calculated, for instance, that energy demands of the whole world could be met by the solar energy falling on a 200-kilometer square plot of ground near the equator. At the present time, however, and solar energy make up only a small fraction of total energy used. See the graph of energy usage in Figure 1-1.
As our knowledge and means of converting these ample energy supplies into usable forms increase we shall be better able to keep pace with rising needs. However, it will take more than just the efforts of science to solve the energy problem. Fuel conservation by all segments of the population must accompany scientific advances.
1.2 Science and Technology Briefly stated, science is the search for relationships that explain and predict the behavior of the universe. Technology is the application of these relationships to our needs and goals.
Finding new energy sources falls into the realm of science. Developing and utilizing these discoveries are matters of technology. The complementary work of scientists and technologists is also referred to, respectively, as research and development. Both government and private industry allocate large sums of money to support the research and development activities of scientists and engineers.
Without such support, there would be little improvement in the processes, products, and services that are derived from these activities. The development of the miniaturized computer is a good example of the result of cooperative effort between science and technology. At the heart of this amazing device is a tiny chip, such as the one shown in Figure 1-2, that contains thousands of transistors. This tiny chip has the calculating capacity of a vacuum tube computer that, twenty-five years ago, needed an entire room for space.
One application of the miniaturized computer is the supermarket electronic checkout. This system reads and records the names and prices of items labeled with the Universal Product Code (UPC). This code, a series of black lines underscored by numbers, is read by a laser beam scanner and the coded information is relayed to the store's computer. This coded information is processed by the computer, which then displays the product's name and price and provides an itemized receipt. While it is recording each purchase, the computer is also checking the store's inventory for reordering purposes.
Computer-assisted instruction demonstrates the application of computers in the field of education. Individualized instruction in any subject is available to the student. The student retrieves the stored information and then converses with the computer by means of a typewriter or television screen.
In banks computers already handle most of the billions of checks Americans write each year. In hospitals computers are used to analyze the medical histories and symptoms of patients as well as to detect and monitor their ailments. A computer program can order prescriptions and alert nurses to administer them at the right time and in the right dosage. A major advance is the use of the computer to assemble thousands of X rays of any part of the body.
In the home computer devices may someday be as commonplace as the kitchen sink. When mass production and technology bring the cost within reach of most routine chores of all kinds can be handled quickly accurately by the computer chip. In cars computers automatically apply the brakes, determine gas efficiency, check the oil and tire pressure; a mini radar can warn the driver of impending collisions.
Many applications of computers still await discovery. High school students who have grown up in our technological society, can meet this challenge and become the engineers and scientists of the future.
1.3 Scientific Laws and Theories In science a law (or ) is a statement that describes a natural event. Unlike that restricting behavior (i.e. traffic laws), scientific laws behavior. For example, Jacques Charles (1746) observed, under certain controlled conditions, a relationship between the temperature and the volume of gases.
This regular behavior of gases, which you will in greater detail in Chapter 8, is now known as law. A scientific law may be stated in words. In physics a law is usually expressed by a math equation relating the natural event to the various permameters that cause it.
A theory is a reasonable explanation of observed events that related. A theory often involves an imaginary model that scientists picture the way an observed event could be. A good example of this is our modern atomic theory, which you will study in Chapters 7, 23, 24, and 25.
Another example is the kinetic molecular theory. In this theory gases are pictured as being made up of many small particles called molecules that are in constant motion. You will study the kinetic molecular theory in Chapter 7.
A useful theory, in addition to explaining past observations, helps to predict events that have not as yet been observed. After a theory has been publicized, scientists design experiments to test the theory. For example, if matter is composed of small particles called atoms, it should behave in a certain way.
If observations confirm the scientists' predictions, the theory is supported. If observations do not confirm the predictions, the scientists must search further. There may be a fault in the experiment, or the theory may have to be revised or rejected.
1.4 Scientific Hypotheses Albert Einstein (1879-1955) was one of the greatest theoretical scientists of all time. Theoretical scientists work mostly with ideas. They use the results of other scientists' observations to develop their theories. Einstein's theories and predictions changed the course of modern science. Once he was asked to explain how a scientist works. "If you want to know the essence of scientific method," he said, "don't listen to what a scientist may tell you. Watch what he does."
Science is a way of doing things, a way that involves imagination and creative thinking as well as collecting information and performing experiments. Facts (observations, principles, laws, and theories) by themselves are not science, but science deals with facts.
The mathematician Jules Henri Poincare (1854-1912) said: "Science is built with facts just as a house is built with bricks, but a collection of facts cannot be called a science any more than a pile of bricks can be called a house."
Most scientists start an investigation by finding out what other scientists have learned about the problem. After the known facts have been gathered, the scientist comes to the part of the investigation that requires considerable imagination.
Possible solutions to the problem are formulated. These possible solutions are called hypotheses. For example, at the time of Johannes Kepler (1571-1630), most scientists believed that the planets move in circular orbits. But the observed movements of the planets could not be satisfactorily explained by circular orbits. So Kepler formulated other hypotheses to explain planetary motion. The one that best fit the observations was: All the planets have elliptic orbits.
In a way, any hypothesis is a leap into the unknown. It extends the scientist's thinking beyond the known facts. The scientist plans experiments, calculations, and observations to test hypotheses.
Without hypotheses, further investigation lacks purpose and direction. In the case of Kepler, he continued his observations and calculations over a period of ten years after the announcement of his hypothesis about elliptic orbits.
Eventually he was able to relate, in the form of an equation, the time required for one orbit of a planet with its distance from the sun. When hypotheses are confirmed, they are incorporated into theories or laws. Thus Kepler's hypotheses have become parts of Kepler's laws of planetary motion.
The scientist's laboratory is any place where an investigation can be conducted. To the astronomer the starry skies are a laboratory. The biologist may do experimental work in a swamp or an ocean. The physicist and chemist are often surrounded by a maze of apparatus housed in buildings designed for specific purposes.
1.5 Certainty in Science In a sense, there is no such as absolute certainty in science. The validity of a scientific conclusion is always limited by the method of observation and, to a certain extent, by the person who made it. If a nurse took a patient's temperature with a thermometer that read 1o too high, the physicians might reach an incorrect conclusion about the condition of the patient.
The validity of every measurement is limited by the precision of the apparatus used. Furthermore, these limits are not always apparent to the experimenter. Galileo Galilei (1564-1642), the famous Italian scientist, thought that the motion of light was instantaneous, since he was unable to measure its speed. Later experiments showed that Galileo's conclusion was wrong.
Hence it is important to keep an open mind about the validity of scientific principles, theories, or hypotheses. No amount of experimentation can ever prove any one of them absolutely, whereas a single crucial experiment can disprove any of them.
When a scientist is unwilling to question a statement in science, no matter how well established it is, that scientist has lost a very important attribute of a successful researcher. For this reason the famous physicist Niels Bohr (1885-1962) told his classes: "Every sentence I utter must be understood not as an affirmation but as a question."
Science has been so successful in answering questions about the physical universe and in making life more pleasant and productive that it has been proposed that the methods that scientists have found successful should be used in other areas as well. This means that the attitudes necessary for successful work in science should be universally productive. Among these attitudes are imagination, a thirst for knowledge, a regard for data and their meaning, a respect for logical thinking, patience in reaching conclusions, and the willingness to work with new ideas.
Ah, the Quest for Knowledge!
There are many topics, however, that do not fall into the realm of science. Among these are questions that concern human motives or the ultimate origin of matter and energy. In general, science deals with repeatable events and with answering "how" rather than "why" questions.
THE CONTENT OF PHYSICS
1.6 Matter A good way to define matter is to describe its various forms. A description is not a definition in the real sense of the word, but it helps to bring an abstract idea down to familiar terms.
Matter can be described in terms of its measurable properties. In describing a person, you might refer to height, weight, eye color, hair color, etc. Similarly, all forms of matter possess properties. And just as a person can be identified by listing various characteristics, so a specimen of matter can be singled out by listing its properties.
The number of properties that can be measured for specimens of matter is very large. Entire handbooks of chemistry and physics are devoted to listings of various properties of matter.
In the study of physics it is important to recognize that unless a property can be measured and compared with some kind of standard, it is of limited use to the scientist. Without measurement there can be no science.
1.7 Mass An important property of matter is its mass. Mass is a measure of the quantity of matter in an object. This is another way of saying that matter takes up space. But the mass of an object cannot be determined merely by measuring its size.
Two objects may have the same size and be composed of the same form of matter, yet one may contain more hollow spaces and thus have less mass. Or, one form of matter may have more mass in the same amount of space than does another form of matter.
For example, a piece of brass and a piece of gold may have the same size but not the same mass. Or, a piece of lead and a piece of aluminum may have the same mass but not the same size.
The mass of an object is determined by comparing it with known masses. Mass measurements will be more fully described in Chapter 2.
1.8 Inertia a The mass of an object can also be measured by using a property of matter called inertia. Inertia is the property of matter that opposes any change in its state of motion.
Inertia shows itself when objects are standing still as well as when they are moving. A baseball resting on the ground will not start moving by itself. A baseball in flight will keep moving unless something stops it. This does not mean that there are two kinds of inertia stationary kind and a moving kind; the same property of matter is merely showing itself in different circumstances.
The inertia of matter can be used to measure mass with a device called an inertia balance, shown in Figure 1-6. One end of the balance is clamped to a table. The pan on the other end can be made to vibrate horizontally. The number of times this device vibrates in a second of time depends upon the length and stiffness of the two supporting metal blades and upon the total mass of the pan and any objects fastened to it. Since the blades vibrate horizontally the action of the inertia balance is entirely independent of gravity, which acts vertically. The more massive the pan and its contents, the slower will be the changes in their motion and the longer will be the time they will take to go through a complete vibration. The time required for a single vibration is called the period.
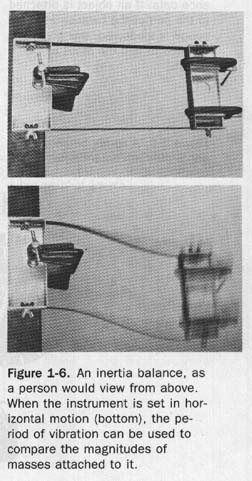
Unknown masses can be measured by comparing their periods with the periods of objects having known masses. First, several of the known objects are used and their periods are plotted as functions of their masses. Then the period of an unknown object is measured and its mass is read from the graph.
The graph may not be a straight line. But by careful analysis of the data the relationship can be expressed by the following equation:
m1/m2 = T2/T1
in which m1 and m2 are two masses (including the mass of the pan in each case) and T1 and T2 are their respective periods.
Masses can also be compared using either a spring balance or a platform balance. For comparing masses, these devices depend upon the pull of the earth's gravity. The stretch of a spring depends upon the amount of pull on the spring. The greater the pull, the greater is the amount of stretch.
Since the pull of gravity is greater on a large mass than it is on a small mass, the spring in a spring balance stretches farther for a large mass than it does for a small mass. The amount of stretch for standard masses can be marked on a scale. The stretch for an unknown mass can then be compared with the stretch produced by the standard mass.
In the case of a platform balance, a uniform beam is balanced at its center on a sharp blade. The blade acts as a pivot. If a mass is added to either side of the beam, the beam tips.
Platforms of equal masses are placed on the beam at equal distances from the pivot. If an unknown mass is placed on one platform (usually the left one), known masses can be added to the other platform until the beam again balances. At that point, the known and the unknown masses are equal.
When the mass of an object is measured by its resistance to a change in its motion, as in the inertia balance, the mass is sometimes referred to as inertial mass.
Mass that is measured with a spring balance or a platform balance, on the other hand, is sometimes called gravitational mass.
Many experiments have shown that the inertial mass and gravitational mass of a substance are always equal, even though they may seem to be unrelated. The single term "mass" can therefore be used to describe both forms.
Now that we have discussed two important properties of matter, we can use these properties to define matter. Matter is anything that has the properties of mass and inertia.
1.9 Mass Density A property of matter that is closely related to mass is mass density. Mass density refers to the amount of matter in a given amount of space and is defined as the mass per unit volume of a substance. Thus if a substance has a mass of 45 g and occupies a space of 15 cm3, its mass density is 3.0 g/cm3 The mathematical equation is
mass density = mass/volume
In giving the mass density of a substance, it is important to include the units (kilograms per cubic meter, grams per cubic centimeter, or some other unit of mass per unit of volume) in order that it may be compared with other values of mass densities.
For example, the mass densities of solids and liquids are usually compared to the mass density of water, while the mass densities of gases are compared to hydrogen. The ratio of such a comparison is called the specific gravity of the substance.
Mass density is a property that varies with the environment. The mass density of a gas increases when the gas is placed under pressure and decreases when the pressure is reduced.
Such quantities as pressure and temperature, which describe the environment of an object, are called conditions. Very often a measurable property of an object will change if the conditions are changed.
1.10 Energy It is said that, as a youngster, James Watt (1736-1819) became interested in steam power by watching water boil in a teakettle over a fire. When he plugged the spout of the kettle, the lid rose. Perhaps this observation eventually led Watt to the improvements of steam engines for which he is famous.
In any case, this observation is a good example of how energy is related to work. We say that work is done when an object is lifted against the pull of the earth's gravity. Later we will define work more completely; for now this common use of the word will be adequate.
We do work when we lift the lid of a teakettle. When we plug the spout, the steam lifts the lid. The work is done by the steam. So we reason that the steam in the kettle had the capacity for doing work before it lifted the lid. The steam had energy.
Energy is the capacity to do work, if the conditions are right. Since energy can often be transformed into mechanical work, the units we use for measuring energy are the same as the units of work.
Energy is sometimes quite noticeable because we have sense organs that are able to detect its presence in various forms. Our eyes respond to visible light energy. Our ears detect sound energy. Special nerves are sensitive to temperature, an indication of heat energy. Other nerves respond to electric energy.
Scientists have discovered forms of energy in addition to those that can be detected by our sense organs. Special detecting and measuring instruments had to be developed to record these forms of energy in a form that we can sense.
For example, we cannot directly sense X rays, a form of energy similar to visible light. But X rays can affect special photographic film. Under visible light, we can look at a piece of exposed and developed X-ray film and tell where the X rays have affected the film.
As another example, we cannot directly sense small amounts of infrared radiation, an invisible form of energy similar to visible light. (We can feel large amounts of infrared radiation as heat.) However, a special thermometer exposed to infrared radiation registers an increase in temperature. We can study the behavior of the thermometer and say that invisible radiation is present.
By means of devices such as these, scientists have extended the range and sensitivity of the human senses. Among the forms of energy that fall into this "extrasensory" category that humans cannot directly detect are chemical energy, gravitational energy, nuclear energy, and forms of energy similar to visible light, such as X rays and infrared rays.
Energy is the concept that unifies physics. More specifically, physicists study and measure the transformation of energy, for the forms of energy are mutually interchangeable. For example, consider the energy you expend when you walk. Traced backward, it came from the food you ate. The food got its energy from nutrients in the ground and radiations from the sun. The sun got its energy from nuclear reactions in its interior, etc. Tracing your walking energy forward, the friction and motion of your feet on the ground heat the ground slightly. This heat radiates into space and helps to evaporate water from the earth, making rain possible, etc.
1.11 Potential Energy Two important forms of energy that an object may have are its energy of position and its energy of motion. Energy of position is potential energy.
Energy of motion is kinetic energy. A book resting on a desk top acquired potential energy when someone did the work of lifting it to the desk top. The book has potential energy, or energy of position, while it is on the desk top.
If the book is pushed off the desk top, it falls to the floor. While falling, it may strike some other object to which it gives some of its energy. This is kinetic energy, or energy of motion.
When the book is stopped by the floor, the rest of its kinetic energy is transformed into other forms of energy, such as sound and heat. In a way, the floor is an arbitrary zero level for our example. If a hole were cut in the floor under the book, the book would continue to fall and do more work when its motion is stopped. For each problem in gravitational potential energy, an arbitrary zero level must be specified.
An object may also have potential energy that is not connected with gravitation in any way. For example, a compressed spring gets its energy from the push that is exerted on it to get it into a compressed position. This energy is called elastic potential energy.
Elastic potential energy is not as easy to compute as the gravitational variety, since one must be familiar with the transformations of various forms of energy. Some of these forms of energy, especially those concerning the interior of the atom, can take the problem to the very frontiers of modern physics.
1.12 Kinetic Energy Every moving object has kinetic energy. This is the same as saying that everything has kinetic energy, because scientists believe that everything in the universe is moving in some way or other.
Since the description of the motion of an object depends on another object in the universe designated as being "motionless," it is necessary to choose an arbitrary zero level or, in this case, an arbitrary stationary point.
In ordinary, earthbound physics the surface of the earth is considered stationary. An object resting on the earth's surface is said to have zero kinetic energy even though the object rotates and revolves with the earth.
1.13 Conservation of Energy There is a constant interplay between potential energy and kinetic energy in physics. Consider the swinging of a mass at the end of a string. When the mass is at the top of its swing, it is momentarily stationary. At that point the energy of the mass is gravitational potential (except for internal energy).
As the mass begins its downward swing, some of the gravitational potential energy changes into kinetic energy. At the bottom of the swing, which we will consider as the zero level for gravitational potential energy, the kinetic energy of the mass is at a maximum because it is moving at its maximum speed.
As the mass swings up the other side of its arc, the energy interchange is reversed. The total amount of energy is always the same it is merely changing form. This discussion demonstrates the law of conservation of energy-- the total amount of energy in a given situation is constant.
The conservation of energy was demonstrated in a striking way by the classical experiments with heat energy conducted by Count Rumford (1753-1814) and James Prescott Joule (1818-1889) in the early part of the nineteenth century.
At that time most scientists thought of heat as a special kind of "fluid" called caloric, which was able to flow in and out of objects without affecting their weights.
Count Rumford was in charge of the boring of brass cylinders for the construction of cannon barrels in Bavaria. Figure 1-11 shows a model of Rumford's apparatus.
During the boring process the barrels became so hot that water poured into them could be brought to a boil. According to the caloric theory, the brass contained a specific amount of caloric. This caloric was being released from the brass as the metal was broken down into chips by the boring tool.
Count Rumford questioned this explanation since the amount of heat produced seemed limitless. As long as the horses provided the work to turn the boring tool, more heat developed.
Rumford demonstrated that if the boring tools were dull and no metal were ground to chips that supposedly contained caloric, heat continued to pour out. In fact, the metal heated up even more. Rumford concluded that the heat produced was a result of the work done on the cannon barrel by the horses; it was not the result of a substance present in the brass.

In spite of Rumford's experiments, the caloric theory existed for fifty more years. Toule continued the investigation of heat by establishing the quantitative relationship between heat and mechanical energy.
This relationship will be discussed more fully in Chapter 9. The equivalence between heat and mechanical energy provided strong evidence that heat is a form of energy. It also supported the theory that the total amount of energy remains constant when it is transformed from one kind to another.
1.14 Relationship between Matter and Energy So far we have discussed matter and energy as though they were two entirely different entities. Actually, the two are inseparably related.
All matter contains some form of energy. Ordinarily, the idea of energy is associated with a substance such as a hot gas or an electrified object. In some cases it is necessary to associate energy with the empty space surrounding an electrified object.
In l905 Einstein expressed the relationship between matter and energy with the following equation:
E= mc2
in which E stands for units of energy (work units), m stands for mass, and c for the speed of light. Einstein developed this equation entirely from theoretical considerations, and there was no way of verifying it in the laboratory at the time.
Subsequent experiments, however, have shown that such a relationship does exist. Einstein's equation states that mass and energy are directly proportional to each other.
When one increases, the other increases; when one decreases, so does the other. The equation is also interpreted to mean that a given amount of mass is equivalent to a specific amount of energy.
A simple example will serve to illustrate the relationship between mass and energy. When you throw a ball, you impart energy to it. Energy is transferred from you to the ball. The mass-energy equation says that since c is constant, the increase in energy E will be accompanied by an increase in mass m.
That is, while the ball is moving, its mass will be greater than it was while the ball was at rest. Both the energy and the mass of the ball have increased. The extra energy and mass of the ball came from you.
When an object is at rest with respect to the observer and the observer's measuring instruments, the mass of the object is said to be the rest mass.
When the object is moving, its mass increases. This new mass, which increases rapidly as the object approaches the speed of light, is called the relativistic mass because it is in keeping with Einstein's theory of relativity.
In the mass-energy equation, m is the relativistic mass. Actually, what Einstein's equation means is that matter is a form of energy and the two terms emphasize different aspects of the same quantity.
This relationship is one of the most important concepts of contemporary physics. It should not be assumed, however, that Einstein's mass-energy equation is a statement of the conservation of matter and energy. The equation has value even if the matter and energy of the universe were constantly changing.
The conservation laws rest on repeated laboratory measurements that show mass and energy are not lost in chemical and physical changes. Some scientists think the law of conservation may not hold for large energies an masses in outer space, but this hypothesis has not been verified.
The deviations predicted by scientists are too small for measurement with present instruments. The relationship between matter and energy will become evident in the study of physics in still another way.
We usually think of light energy in terms of waves and of matter in terms of particles. Actually, there are times when light acts as though it has granular properties. In other words, there are aspects of light that can be explained only by assuming that it is made up of discrete particles. Similarly, particles of matter exhibit wave properties. This wave-particle duality of nature is a key as well as a puzzle in physics, and scientists admit that they are far from a complete understanding of it.
Perhaps you will play a role in unlocking this fundamental secret of nature. Ah Yaz!
1.15 Subdivisions of Physics The study of the physical universe is called physical science. Chemistry is a physical science. It deals with the composition of matter and reactions among various forms of matter. Physics is also a physical science. It is concerned with the relationship between matter and energy. The ultimate goal of physics is to explain the physical universe in terms of basic interactions and simple particles.
Since both chemistry and physics deal with matter, these two sciences overlap to some extent, but a distinguishing consideration in the study of physics is always the idea of energy, what it is, how it affects matter and how matter affects it, and how it can be changed from one form to another.
The study of physics can be subdivided in a number of ways. The sequence of topics followed in this book is one that has been used for many years because of its logical progression and because it leads from concepts developed many years ago to those at the very frontiers of physics.
The chapters in your text fall into four major categories: mechanics and heat, waves, electricity, and nuclear physics.
The first category includes the study of motion, forces, work, power, and certain aspects of heat. These topics, and the ways in which they involve the transfer of energy, are considered in Chapters 3 through 9. (Chapter 2 describes measurement and problem solving, which are essential to the entire subject of physics.)
Waves are considered in Chapters 10 through 15.
Sound energy and light energy are transmitted as waves. The nature of waves as a mechanism for energy transfer and the characteristics of sound and light comprise the subject matter of this section of your text.
Electricity involves a form of energy that is increasingly important to our way of life. In Chapters 16 through 22, the ways of generating and transmitting this vital commodity are explained. This section of the text also contains an introduction to the study of electronics, which is the basis for radio; television, and computers.
In the last three chapters of the book, the principles of nuclear and particle physics are introduced. It is here that the physicist tries to get to the very heart of the composition of matter and of the energy forms that bind the building blacks of the universe together.
With topics ranging from "atom smashers" to the most distant star systems, this part of the course is usually called modern physics. The authors hope that as you reach this final section of the course, you will have caught the excitement of physics, a subject that features unlimited topics and possibilities.
SUMMARY
The world's growing population requires ever-larger amounts of energy. By discovering and developing new energy sources, science and technology can help to meet this need. Science and technology are also called research and development. The miniaturized computer and its many applications are good examples of the impact of science and technology on our way of life.
Even so, many applications of computers still await discovery. Scientific laws, theories, and hypotheses are steps in the search for truth in the physical universe. A hypothesis is a possible solution to a scientific problem. Confirmed hypotheses become laws or theories, but even these are never absolutely certain.
Science deals with the "how" but not the "why" of the universe. Among the properties of matter are mass (the measure of the quantity of matter), inertia (the resistance of matter to change in its state of motion), and mass density (mass per unit volume). The environment of a sample of matter is called a condition.
Physics further deals with the various types of energy and their transformations. Energy is the capacity for doing work.
Potential energy is energy of position. Gravitational energy and elastic energy are forms of potential energy. Kinetic energy is energy of motion. Potential and kinetic energy are readily interchanged. However, the law of conservation of energy states that energy may be changed from one form to another without loss.
The experiments of Count Rumford and James Prescott Joule verified this law. Einstein's relativity equation, E = mc2, states that energy and mass are directly proportional to each other and that they are interchangeable.
Mass can thus be considered to be a form of energy. Physics is the physical science that deals with various. forms of energy. Its four main subdivisions are mechanics and heat, wave motion, electricity, and nuclear and particle physics. The last of these is also referred to as modem physics.
VOCABULARY
caloric, conditions, energy, hypothesis, inertia, kinetic energy, law, law of conservation of energy, mass, mass density, matter, period, physics, potential energy, relativistic mass, rest mass, science, specific, gravity, technology, theory
Ah Yaz Indeed!
 Assignment Sheet for this Research Text Only.
Assignment Sheet for this Research Text Only.
 Go to Textbook Assignments for Portfolio:
Go to Textbook Assignments for Portfolio:
.................................First Semester
.................................Second Semester