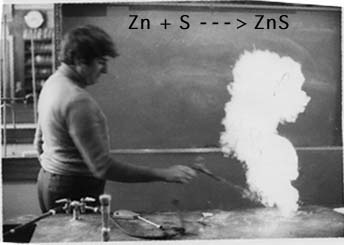
Zinc Plus Sulfur Reaction

This is a great, exothermic, dangerous demonstration of Two Elements combining to form a compound.
Use the equation Zn + S ---> ZnS to calculate the reaction ratios of Zn and S.
The Atomic Mass of Zn is 65 g/mol and S is 32 g/mol.
From this we see that we need twice as many grams of Zn as S.
If you do not wish to make the evening news, use small quantities. I recommend the following:
In a 100 ml beaker, place 5 grams of Powdered Sulfur (Flowers of Sulfur) with 10 grams of Powdered Zinc.
Point out that this is a heterogeneous mixture. Not good for intimate contact of molecules.
Mix well and show that it is now a homogeneous mixture, ready for reacting.
Show also that it is still a mixture, not a compound. It can be separated by physical processes.
Place the mixture on a brick. Much heat will be formed.
Have everyone move back at least three meters, the flaming sparks do fly!
Don your Goggles, Pith Helmet, and Lab Coat.

Have a Fire Extinguisher close at hand.
Take a Bunsen Burner, and it's GAS ON, IGNITE!
Review Activation Energy, Endothermic and Exothermic reactions.
With a window pole use the burner to start the reaction.
Be ready to dodge the sparks! Be sure the reaction is over before approaching. There will be after bursts of reaction.
Show the students that the product of the reaction is no longer Zinc nor Sulfur. It's a new substance, the compound Zinc Sulfide.
To dispose of it, scrape into a trash can after it cools off.